Abstract
The term ‘baboon syndrome’ was introduced in 1984 to describe a special form of systemic, contact-type dermatitis that occurs after ingestion or systemic absorption of a contact allergen in individuals previously sensitized by topical exposure to the same allergen in the same areas. Its clinical picture presents as an erythema of the buttocks and upper inner thighs resembling the red bottom of baboons. This reaction was originally observed with mercury, nickel, and ampicillin. In 2004, some authors proposed the acronym SDRIFE standing for ‘symmetric drug-related intertriginous and flexural exanthema’ specifically for cases elicited by systemically administered drugs. Since 1984, about 100 cases have been reported in the literature; for most of the concerned drugs, previous skin sensitization or possible cross-sensitization has not been shown.
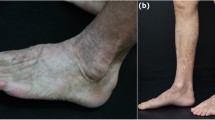
We report the first case of SDRIFE due to rivastigmine, with the exception of an erythematous maculopapular eruption due to rivastigmine that was previously reported. Rivastigmine is a reversible and noncompetitive acetylcholinesterase inhibitor used for the treatment of Alzheimer disease.
SDRIFE is an important condition to keep in mind in order to avoid a misdiagnosis when dealing with other exanthematous disorders and to prevent re-exposure to the responsible allergen in the future.
Similar content being viewed by others
References
Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemicallyinduced allergic contact dermatitis. Contact Dermatitis 1984; 10: 97–100
Sánchez-Morillas L, Reaño Martos M, Rodríguez Mosquera M, et al. Baboon syndrome. Allergol Immunopathol (Madr) 2004; 32: 43–5
Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis 2004; 51: 297–310
Helmbold P, Hegemann B, Dickert C, et al. Symmetric ptychotropic and nonpigmenting fixed drug eruption due to cimetidine (so-called baboon syndrome). Dermatology 1998; 197: 402–3
Barbaud A, Tréchot P, Granel F, et al. A baboon syndrome induced by intravenous human immunoglobulins: report of a case and immunological analysis. Dermatology 1999; 199: 258–60
Chowdhury MM, Patel GK, Inaloz HS, et al. Hydroxyurea-induced skin disease mimicking the baboon syndrome. Clin Exp Dermatol 1999; 24: 336–7
Audicana M, Bernedo N, Gonzalez I, et al. An unusual case of baboon syndrome due to mercury present in a homeopathic medicine [letter]. Contact Dermatitis 2001; 45: 185
Loze I, Milpied-Homsi B, Parent E, et al. The baboon syndrome, a particular cutaneous drug eruption: four case reports [in French]. Nouvelles Dermatologiques 2001; 20: 624–5
Monastero R, Lopez G, Mannino M, et al. Erythematous maculopapular eruption due to rivastigmine therapy. Am J Med 2001; 111: 583–4
Schmutz JL, Barbaud A, Tréchot P. Baboon syndrome: frontline new for the backside (letter) [in French]. Ann Dermatol Venereol 2001; 128: 1378
Isaksson M, Ljunggren B. Systemic contact dermatitis from ethylenediamine in an aminophylline preparation presenting as the baboon syndrome. Acta Derm Venereol 2003; 83: 69–70
Gallo R, Parodi A. Baboon syndrome from 5-aminosalicylic acid [letter]. Contact Dermatitis 2002; 46: 110
Amichai B, Grunwald MH. Baboon syndrome following oral roxithromycin [letter]. Clin Exp Dermatol 2002; 27: 523
Sánchez-Morillas L, Reaño Martos M, Rodríguez Mosquera M, et al. Baboon syndrome due to pseudoephedrine [letter]. Contact Dermatitis 2003; 48: 234
Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its parthomechanism. Dermatol Online J 2003; 9: 2
Moreno-Ramírez D, García-Bravo B, Rodríguez Pichardo A, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol 2004; 21: 250–3
Jankowska-Konsur A, Kolodziej T, Szipietowski J, et al. The baboon syndrome: report of two first cases in Poland. Contact Dermatitis 2005; 52: 289–90
Armingaud P, Martin L, Wierzbicka E, et al. Baboon syndrome due to a polysensitization with corticosteroids [in French]. Ann Dermatol Venereol 2005; 132: 675–7
Dhingra A, Grover C. Baboon syndrome [letter]. Indian Pediatr 2007; 44: 934
Arnold AW, Häusermann P, Bach S, et al. Recurrent flexural exanthema (SDRIFE or baboon syndrome) after administration of two different lodinated radio contrast media. Dermatology 2007; 214: 89–93
Garcia-Menaya JM, Cordobés-Durán C, Bobadilla P, et al. Baboon syndrome: 2 simultaneous cases in the same family. Contact Dermatitis 2008; 58: 108–9
Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol 2009; 34: 355–7
Barbaud A. Global management of cutaneous adverse drug reactions [in French]. Ann Dermatol Venereol 2007; 134: 391–401
Häusermann P, Bircher AJ. SDRIFE: another acronym for a distinct cutaneous drug exanthema: do we really need it? Dermatology 2007; 214: 1–2
Bryant CA, Ouldred E, Jackson SH, et al. Purpuric rash with donazepil treatment. BMJ 1998; 317: 787
Herfs H, Schirren CG, Przybilla B, et al. Baboon syndrome: a particular manifestation of hematogenous contact reaction [in German]. Hautarzt 1993; 44: 466–9
Weiss JM, Mockenhaupt M, Schopf E, et al. Reproducible drug exanthema to terbinafine with characteristic distribution of baboon syndrome [in German]. Hautarzt 2001; 52: 1104–6
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–45
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allain-Veyrac, G., Lebreton, A., Collonnier, C. et al. First Case of Symmetric Drug-Related Intertriginous and Flexural Exanthema (SDRIFE) Due to Rivastigmine?. Am J Clin Dermatol 12, 210–213 (2011). https://doi.org/10.2165/11318350-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11318350-000000000-00000