Abstract
Fluarix® is a trivalent, inactivated, split-virion influenza vaccine containing 15 μg haemagglutinin from each of the three influenza virus strains (including an H1N1 influenza A virus subtype, an H3N2 influenza A virus subtype and an influenza B virus) that are expected to be circulating in the up-coming influenza season.
Fluarix® is highly immunogenic in healthy adults and elderly, and exceeds the criteria that make it acceptable for licensure in various regions (including the US and Europe). In a large, phase III, placebo-controlled, double-blind trial conducted in the US (2004/2005) in subjects aged 18–64 years, postvaccination sero-conversion rates against the H1N1, H3N2 and B antigens were 60–78% and respective postvaccination seroprotection rates were 97–99% in Fluarix® recipients. Another phase III trial conducted in the US (2005/2006) established the noninferiority of Fluarix® versus another trivalent inactivated influenza virus vaccine in subjects aged ≥18 years, including a subgroup of elderly subjects. In annual European registration trials, Fluarix® has consistently exceeded the immunogenicity criteria set by the EU Committee for Medicinal Products for Human Use for adults and the elderly. Fluarix® demonstrated immunogenicity in small, open-label studies in at-risk subjects.
During a year when the vaccine was well matched to the circulating strain, Fluarix® demonstrated efficacy against culture-confirmed influenza A and/or B in a placebo-controlled trial in adults aged 18–64 years. In addition, Fluarix® vaccination of pregnant women demonstrated efficacy in reducing the rate of laboratory-confirmed influenza in the infants and reducing febrile respiratory illnesses in the mothers and their new-born infants in a randomized trial.
Fluarix® was generally well tolerated in adults and the elderly in well designed clinical trials and in the annual European registration trials, with most local and general adverse events being transient and mild to moderate in intensity. The most common adverse reactions in recipients of Fluarix® were pain, redness or swelling at the injection site, muscle aches, fatigue, headache and arthralgia.
In conclusion, Fluarix® is an important means of decreasing the impact of seasonal influenza viruses on adults and the elderly.
Similar content being viewed by others
1. Introduction
Influenza is a contagious viral infection of the respiratory tract which is easily transmitted from person to person via respiratory droplets expelled during coughing or sneezing, from person to person via direct contact, or when someone touches a surface contaminated with the virus.[1–3] The influenza infection is characterized by respiratory symptoms such as cough and sore throat, and by more general symptoms such as fever, headache and malaise. Influenza can exacerbate underlying medical conditions (e.g. pulmonary or cardiac diseases) or cause secondary bacterial pneumonia, sinusitis or otitis media or primary influenza viral pneumonia.[3–5] Influenza infection has also been associated with encephalopathy, transverse myelitis, myositis, myocarditis and pericarditis. Influenza is generally debilitating, and often results in several days of restricted activity and lost school or work time, and may result in hospitalization.[3,5–7] Most morbidity and mortality occurs in the elderly and those with underlying risk factors such as chronic cardiorespiratory disease, immunosuppression or diabetes mellitus.[3,8]
The viruses causing influenza belong to the Orthomyxoviridae family.[2,9] These viruses are enveloped, single-stranded, negative-sense RNA viruses and can be classified into types A, B and C, depending on the antigenic differences of their structural proteins.[2,9–12] Influenza A and B viruses are responsible for the yearly epidemic outbreaks of respiratory illness in humans. The 11 proteins of influenza A and B viruses are encoded by eight gene segments. Haemagglutinin and neuraminidase are expressed on the surface of the virus and are, respectively, required for entry to and release from the host cells.[2,9,13] The influenza A virus can infect a wide variety of avian and mammal species, with numerous haemagglutinin and neuraminidase influenza A virus subtypes (e.g. H1N1, H3N2) having been identified.[9,11] The influenza B virus, whose host is generally humans, has a single haemagglutinin and neuraminidase type, and is not categorized into subtypes. Currently circulating influenza B viruses can be separated into two distinct genetic lineages (Yamagata and Victoria). Infections caused by influenza C are sporadic and usually of little clinical importance.[3]
The influenza RNA polymerase lacks a proof-reading mechanism and mutations in the genes of the influenza virus are common.[8] Minor antigenic changes due to random point mutations (antigenic drift)[14] are responsible for annual influenza epidemics, with the changes in haemagglutinin and neuraminidase helping the virus to overcome the immune response generated in a host population through prior infection or vaccination. Antigenic shifts are responsible for influenza pandemics (global outbreak of the disease).[15–19] Pandemics can be mild or severe in terms of the illness and death they cause.[17,18] At the genetic level, pandemic influenza viruses may arise through genetic reassortment (in which genes from animal and human influenza viruses mix together to create a human-animal influenza reassortant virus) or by genetic mutation (in which genes in an animal influenza virus change allowing the virus to infect humans and efficiently transmit from human to human).[15,16,19]
Influenza epidemics usually occur each year, generally during the winter months in temperate regions: October to April in the Northern Hemisphere and May to September in the Southern Hemisphere.[2,3] Influenza activity occurs throughout the year in tropical regions. The WHO, assisted by National Influenza Centres, identifies prevalent circulating strains relevant to the Northern or Southern Hemispheres.[20] Two influenza A strains (one H1N1 and one H3N2) and an influenza B strain are recommended for vaccine inclusion. The antigenic composition of the influenza vaccine needs to be evaluated each year as new influenza variant strains may have emerged since the previous influenza season. Consequently, the trivalent inactivated influenza vaccines are reformulated nearly every year.
Both inactivated and live attenuated influenza vaccines are available (see section 6 for further details).[4] Fluarix® is a trivalent, inactivated, split-virion vaccine containing 45 μg of haemagglutinin (15 μg of each of the haemagglutinins of the three virus strains that are expected by the WHO to be circulating in the community in the upcoming winter). It has been manufactured in Germany since 1987 and is available worldwide in over 100 countries. The influenza strains used in the preparation of Fluarix® for the winter season in the Northern and Southern Hemisphere in recent years are shown in tables I and II; these strains met the recommendations of the WHO.
Each of the three strains of influenza virus contained in Fluarix® are produced and purified separately.[22] Embryronated hen’s eggs are inoculated with the respective virus and the virus is allowed to replicate in the allantoic fluid. After harvesting, the allantoic fluid from the eggs containing the cultured virus is concentrated and purified by zonal centrifugation and detergent is used to disrupt (split) the virus. Following further purification, each influenza virus solution is then inactivated. Fluarix® is formulated from each of the three split inactivated virus solutions, without preservatives, without adjuvants and without thimerosal.
This review focuses on the immunogenicity and reactogenicity of the trivalent, inactivated, split-virion influenza vaccine Fluarix® (also known as Influsplit SSW® [Germany] and α-RIX™ [Belgium]) in adults and the elderly.
2. Immunogenicity
Immunogenicity data reviewed in this section have been obtained from published literature, the US manufacturer’s prescribing information[22] and the manufacturer’s clinical trial registry.[23]
Unless otherwise stated, subjects enrolled in the studies reviewed in this section received a single 0.5 mL dose of Fluarix® administered by intramuscular injection in the upper arm. Each dose of the vaccine contained 15 μg of each of the three haemagglutinin antigens from the three virus strains recommended by the WHO for the season and hemisphere in which it was administered (see tables I and II).
Immunogenicity was determined by assessing the level of haemagglutination-inhibiting (HI) antibodies against all three haemagglutinin antigen components of the vaccine in the serum of blood taken just before (prevaccination; day 0) and after (postvaccination; generally day 21) administration of the vaccine.
Primary immunogenicity endpoints included:
-
geometric mean titre (GMT) of HI antibodies;
-
seroconversion rates defined as the proportion of subjects with either an HI titre increase from <1 : 10 prevaccination to ≥1 : 40 postvaccination, or the proportion of subjects with a prevaccination titre ≥1 : 10 and a ≥4-fold increase in postvaccination antibody titre;
-
seroprotection rates defined as the percentage of subjects with a postvaccination HI titre ≥1 : 40.
The immunogenicity endpoints of the studies were evaluated in light of the immunogenicity criteria for influenza vaccines set by the European Medicines Agency Committee for Medicinal Products for Human Use (CHMP)[24] and/or the US FDA Center for Biologics Evaluation and Research (CBER)[2] [see table III].
Immunogenicity criteriaa for adults (aged 18–60[24] or 18–64[2] years) and the elderly (aged >60[24] or >65[2] years) set by the Committee for Medicinal Products for Human Use (CHMP) for the European Medicines Agency[24] or the US FDA Center for Biologics Evaluation and Research (CBER).[2] For the vaccine to be considered sufficiently immunogenic at least one of the three criteria have to be met for each of the antigenic strains (CHMP) or the lower limit of the 95% CI should be met for both criteria (CBER)
Analyses were performed on the total vaccinated cohort and the according-to-protocol (ATP) cohort for immunogenicity. The ATP cohort for immunogenicity included all subjects who met all eligibility criteria, complied with the procedures defined in the protocol and for whom data concerning immunogenicity endpoint measures were available.
2.1 Kinetics of Humoral Antibody Response
The immune response to a single dose of Fluarix® is rapid.[25,26] Seroprotection rates against the virus strains were 85%, 71% and 94% against H1N1, H3N2 and B antigens, respectively, 1 week after vaccination (1993 season) with Fluarix® in a noncomparative study in 48 adult subjects with prevaccination seroprotection rates of 52%, 21% and 65%, respectively.[25] These subjects had not previously received an annual influenza vaccination.
The antibody response is persistent.[26–28] In an open-label, registration study (Northern Hemisphere; 2000/2001) that investigated the persistence of the antibody titre in 110 Fluarix® recipients aged ≥18 years, the antibody titres against each of the three antigens of the vaccine followed a similar pattern over time, peaking at 3 weeks post-vaccination and slowly declining thereafter (figure 1).[27] However, GMTs remained well above 40 (the level that provides protection in at least 50% of recipients[24,29]) at all timepoints up to 27 weeks (figure 1).[27] After 12 months (data available from a poster and abstract[28]), the GMTs for the still accessible study population (n = 95) were 96 (95% CI 75, 122), 66 (95% CI 50, 86) and 111 (95% CI 89, 140) for antibodies against H1N1, H3N2 and B antigens, respectively.
Geometric mean titres (GMTs) of the three antigen strains of the 2000/2001 Fluarix® vaccine before vaccination (0) and 3, 11, 19 and 27 weeks postvaccination in recipients of the vaccine (a) aged 18–60 years (n = 57) and (b) aged >60 years (n = 53).[27] The dashed horizontal lines indicate the level that provides protection in at least 50% of recipients.[24] Reproduced from Hehme et al.,[27] with permission from Adis, a Wolters Kluwer business (© Adis Data Information BV [2002]). All rights reserved.
2.2 In Adults
2.2.1 Placebo-Controlled Trials
A phase III, randomized, double-blind, placebo-controlled trial (table IV) conducted in the US (2004/2005) demonstrated the immunogenicity of Fluarix® in adult subjects aged 18–64 (mean 39.1) years.[23,30] A total of 935 subjects were included in the ATP immunogenicity analysis. Postvaccination seroconversion rates were 60–78% and postvaccination seroprotection rates were 97–99% in Fluarix® recipients (co-primary endpoints). The vaccine exceeded the two preset immunogenicity criteria that made it acceptable for use as a vaccine against influenza in the US (table III); namely, that the lower limit of the two-sided confidence interval (CI) for the postvaccination seroprotection rate for each of the three viral strains was ≥70%, and that the lower limit of the two-sided 95% CI for the postvaccination seroconversion rate at day 21 for each of the three viral strains was ≥40% (table IV).
Immunogenicity of a trivalent inactivated split-virion, seasonal influenza vaccine (Fluarix®; FLU). In the double-blind, placebo (PL)-controlled, multicentre trials,[30–32] healthy subjects aged 18–64 years were randomized to FLU or PL. A phase III, single-blind, active-comparator, multicentre trial evaluated the noninferiority of FLU vs Fluzone® (FLZ; another US-licensed trivalent inactivated influenza vaccine) in subjects aged 18–95 years.[33] Additional data relating to these trials have been obtained from the manufacturer’s clinical trial registry[23] and the manufacturer’s prescribing information.[22] The antigenic composition of the FLU and FLZ vaccines met the WHO recommendations for the preparation of the influenza vaccine for the season and hemisphere in which they were administered (table I). Subjects received a single intramuscular 0.5 mL dose of the vaccine. Serum haemagglutination inhibition (HI) antibody titres and derived variables were evaluated prevaccination on day 0 and postvaccination on day 21. Data from the according-to-protocol (ATP) cohort for immunogenicity are presented
Two other randomized, double-blind, multicentre trials (conducted in the Czech Republic[31,32] and Finland[31]) in 2005/2006[32] and 2006/2007[31] that immunized a total of 7652[31] and 6203[32] healthy adult subjects aged 18–64 years also demonstrated the immunogenicity of Fluarix®. Immunogenicity was only assessed in a subgroup (n = 947[32] and 439[31]) of adults from these trials, as the assessment of efficacy (see section 3), rather than immunogenicity, was the primary endpoint. Seroconversion rates (74–89%; table IV) and seroprotection rates (87–98%; table IV) in Fluarix® recipients in these trials also exceeded CBER and CHMP acceptability criteria (table III).
2.2.2 Noninferiority Trial
The noninferiority of Fluarix® versus another US-licensed trivalent inactivated influenza virus vaccine (Fluzone®) was established in a phase III, single-blind, multicentre trial conducted in the US (2005/2006) that randomized 1845 subjects aged 18–95 years to treatment (data obtained from an abstract,[33] the manufacturer’s clinical trials registry[23] and the US manufacturer’s prescribing information).[22,23,33] Noninferiority of Fluarix® versus Fluzone® was demonstrated based on the GMTs and seroconversion rates (co-primary endpoints). The adjusted GMT ratio of Fluzone® : Fluarix® was 0.65 (95% CI 0.58, 0.73) for H1N1, 0.93 (95% CI 0.83, 1.04) for H3N2 and 1.13 (95% CI 1.03, 1.25) for the B viral strain. The between-group difference of the seroconversion rate of Fluzone® minus Fluarix® was −0.12 (95% CI −0.16, −0.07) for H1N1, −0.02 (95% CI −0.06, 0.03) for H3N2 and 0.01 (95% CI −0.04, 0.06) for the B viral strain. Noninferiority was established since the upper limit of the two-sided 95% CI for the adjusted GMT ratios of Fluzone® : Fluarix® was ≤1.50 for each of the H1N1, H3N2 and B viral strains and the upper limit of the two-sided 95% CI for the between-group difference of the seroconversion rate of Fluzone® minus Fluarix® was ≤10% for each viral strain. The seroconversion rates and seroprotection rates for Fluarix® in this trial also exceeded CBER and CHMP acceptability criteria (table III) for all three viral antigens (table IV) for adults aged ≥18 years.
2.2.3 Annual Registration Trials
Annual registration studies conducted in Europe between 1992 and 2009 stratified the healthy participants according to age; adults aged 18–60 years and the elderly (aged >60 years; see section 2.3).[23,27,34] As mandated by the European Medicines Agency guidelines, >50 subjects were included in each of these age groups. Most registration studies were conducted in Germany, and since 1998 the studies were conducted in June or July of the same year of registration. Primary endpoints included GMTs of HI antibodies, seroprotection rates (day 0 and 21), seroconversion rates (day 21) and seroconversion factors (day 21).
In the last 15 years, Fluarix® has met all three CHMP immunogenicity criteria (table III) for each virus strain in adults aged 18–60 years (see figure 2).
Immunogenicity of Fluarix® (FLU) in noncomparative, annual registration studies (Northern Hemisphere) in healthy adult subjects aged 18–60 years. Seroconversion rates at day 21 (a); seroprotection rates at day 21 (b); and seroconversion factors at day 21 (c) [see table III for definitions] were assessed in the according-to-protocol cohort (n = 50–59) after an intramuscular administration of a 0.5 mL dose of FLU (1992–2009). No annual registration trial was conducted in the 2003 season, as the vaccine composition was identical to that of 2002. The antigenic composition of FLU met the WHO recommendation for the preparation of the influenza vaccine for the season and hemisphere in which it was administered (see table I). The dashed horizontal lines indicate the lower limit set by the Committee for Medicinal Products for Human Use for the European Medicines Agency. Data were obtained from the manufacturer’s Clinical Trial Registry.[23]
2.3 In the Elderly
2.3.1 Comparison with Other Vaccines
Fluarix® is immunogenic in elderly subjects and exceeds the regulatory criteria set by the CHMP and CBER, according to data from a randomized, single-blind, active-comparator, multicentre trial (2005/2006; see section 2.2.2 for details of all subjects enrolled in the trial) that included a subgroup of 1120 subjects (ATP cohort) aged ≥65 years.[23,33] In this trial (see table IV), the immunological noninferiority of Fluarix® versus Fluzone® was established, based on seroconversion rates and GMTs for each of the three antigens contained in the vaccine (see section 2.2.2).[22,23,33] GMTs postvaccination were lower in the subgroup of elderly subjects than those in younger subjects aged 18–64 years enrolled in the trial.[22]
Fluarix® also exceeded the CHMP criteria for immunogenicity in the elderly (see table III) in a randomized, open-label trial (2002/2003 season) in 840 subjects aged ≥60 years who had not been vaccinated or diagnosed with influenza in the previous season.[35] In this trial, the immunogenicity of Fluarix® was compared with that of an MF59-adjuvanted subunit vaccine (Fluad®) and a virosome-based subunit vaccine (Inflexal V®; Infectovac Flu® [in Germany]).[35] As with the other two vaccines, at 4 weeks postvaccination, Fluarix® exceeded the CHMP acceptability criteria for the elderly (see table III) for immunogenicity with respect to seroconversion rates (74.8%, 67.4% and 78.0%), seroprotection rates (93.8%, 90.1% and 91.2%) and seroconversion factors (17.4, 11.6 and 13.2) for each of the three antigens contained in the vaccine.
2.3.2 Noncomparative Trials
Annual Registration Trials
Annual registration studies carried out between 1992 and 2009 in subjects aged >60 years indicate that Fluarix®, with a few exceptions (1996 and 1997), met or exceeded all three immunogenicity criteria for the elderly set by the CHMP (table III) for each of the three antigens contained in the vaccine (figure 3).[27,34] Seroconversion rates were lower than those set by the CHMP for the H1N1 (in 1996 and 1997) or H3N2 (in 1997) antigens due to high prevaccination GMTs. However, seroprotection rates were high in these years (95–100%).
Immunogenicity of Fluarix® (FLU) in annual registration studies (Northern Hemisphere) in healthy elderly subjects aged >60 years. Seroconversion rates at day 21 (a); seroprotection rates at day 21 (b); and seroconversion factors at day 21 (c) [see table III for definitions] were assessed in the according-to-protocol elderly cohort (n = 50–59) after the intramuscular administration of a 0.5 mL dose of FLU (1992–2009). No annual registration trial was conducted in the 2003 season, as the vaccine composition was identical to that of 2002. The antigenic composition of FLU met the WHO recommendations for the preparation of the influenza vaccine for the season and hemisphere in which it was administered (see table I). The dashed horizontal lines indicate the lower limit set by the Committee for Medicinal Products for Human Use (CHMP) for the European Medicines Agency. Data were obtained from the manufacturer’s Clinical Trial Registry.[23]
In the annual registration studies, seroconversion factors were generally lower in elderly subjects (figure 3) than those in younger adults (figure 2), but, nevertheless, exceeded those set by the CHMP for the elderly.[23]
Institutionalized Elderly
The immunogenicity of the Fluarix® vaccine was also established in institutionalized elderly (aged 65–100 years; mean age 80 years) with multiple co-morbid conditions in a noncomparative, multicentre trial.[36] In the 457 elderly who were included in the immunogenicity evaluation, seroconversion rates (47–74%), seroprotection rates (60–93%) and seroconversion factors (4.2–7.9) at day 28 postvaccination all exceeded the CHMP immunogenicity criteria for the elderly (table III) for each of the three vaccine antigens.
2.4 At-Risk Populations
The immunogenicity of Fluarix® generally exceeded the CHMP criteria set for healthy adults (see table III) for each of the three viral antigens in small, noncomparative studies in at-risk subjects (table V).[27,37–39] The studies in which CHMP criteria were met included patients with type 1 diabetes,[27] cancer,[27,37] kidney transplant,[27] liver transplant,[38] splenectomy[39] or chronic obstructive pulmonary disease.[27] Two studies in which all three of the CHMP criteria were not met included a study in patients with haemato-oncological disorders aged 22–84 years who were receiving chemotherapy[40] and a study in patients with systemic lupus erythematosus or rheumatoid arthritis who were receiving immunosuppressive therapy.[41] Both studies enrolled small numbers of patients (≤20) in each risk group (table V).
Immunogenicity of Fluarix® in at-risk adults (aged ≥18 years) enrolled in noncomparative trials. The antigenic composition of the Fluarix® vaccine met the WHO recommendations for the preparation of the influenza vaccine for the season in which it was administered (see table I). Subjects received a single 0.5 mL dose of the vaccine administered intramuscularly. Serum haemagglutination inhibition antibody titres and derived variables were evaluated prevaccination on day 0 and postvaccination on day 21,[27,38] 28[37,39,40] or 30[41]
2.5 Cell-Mediated Immunity
Fluarix® administration has been associated with an increase in cell-mediated immunity.[42–44] For example, in 23 patients scheduled for tonsillectomy, intramuscular administration of Fluarix® (2000/2001) significantly increased the numbers of influenza virus-specific antibody-secreting cells for the H1N1 (p = 0.0011), H3N2 (p = 0.017) and B (p = 0.022) viral strains in peripheral blood after 1 week.[43] The numbers of influenza virus-specific antibody-secreting cells were also higher in tonsils (p = 0.014 for H1N1 and p = 0.0032 for H3N2), but not in the nasal mucosa, in this vaccinated group compared with those reported in another study[45] of nonvaccinated volunteers. Similarly, in another study,[44] intramuscular Fluarix® (2002/2003) administration induced mucosal and systemic T-cell responses in both the palatine tonsils and blood in ten healthy subjects. Another study (presented as an abstract) in 24 healthy individuals administered Fluarix® (2006/2007) demonstrated that, although mean white blood cell counts and mean absolute lymphocyte counts remain similar before and after vaccination, the expression of CD4+CD25+ regulatory T cells increased after vaccination (p < 0.05 vs prevaccination).[42]
2.6 Cross Immunogenicity
A limited number of small studies have investigated the cross immunogenicity of Fluarix®.[46–48]
In one study, the sera of subjects (n = 21–46) aged 18–60 years immunized with Fluarix® during the seasons 1997/1998, 1998/1999 and 2003/2004 were tested against subsequent drift variants of influenza A and B viruses.[48] A high degree of cross immunogenicity against subsequent homologous drifts of influenza A/H1N1 (seroprotection rates of 73–86%) and influenza B (seroprotection rates of 95%) was observed. However, in terms of the genetically more variable component influenza A/H3N2, Fluarix® demonstrated lower rates of seroprotection against some of the subsequent drift variants (38–96%). Similar outcomes were observed in recipients of the vaccine who were aged >60 years.
In one study conducted in Italy in 2005, microneutralization assays against the avian influenza (H5N1) strain A/Hong Kong/156/97 were conducted on sera from healthcare workers (n = 42) who had been vaccinated with Fluarix®. Postvaccination (30 days), 34% of the study population demonstrated a 20-fold increase of neutralizing antibodies against H5N1.[46] However, in another small pilot study (2005/2006) conducted in China, six recipients of Fluarix® and four recipients of another split virion inactivated influenza vaccine were tested for inhibitory activity against the avian influenza (H5N1) virus strains A/Hong Kong/483/97 and A/Thailand/1(KAN-1)/04 using HI antibody and microneutralizing assays at 1, 3 and 6 months postvaccination.[47] No cross-reactivity to these avian strains were detected at any timepoint.
2.7 Coadministration with Other Vaccines
Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine (Boostrix®) and Fluarix® vaccine may be administered together without compromising the immunogenicity of either vaccine, according to a randomized, open-label, multicentre trial in 1497 adult subjects aged 19–64 years.[49] Subjects were randomized to either the concomitant administration of Fluarix® and Tdap or the sequential administration of the vaccines (Fluarix® on day 0 followed by Tdap administration 1 month later) [see table VI for further study design details].
Concomitant administration of an influenza vaccine (Fluarix®; FLU) and a tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Boostrix®; Tdap). In an open-label, multicentre trial in adult subjects aged 19–64 years,[49] subjects were randomized to either the concomitant administration of FLU and Tdap (FLU + Tdap; n = 748) or the sequential administration of the vaccines with FLU administered on day 0 followed by Tdap 1 month later (FLU→ Tdap; n = 749). The study was conducted in the US. A 0.5 mL dose of Tdap contained 2.5 Lf diphtheria toxoid, 5 Lf tetanus toxoid, 8 μg pertussis toxoid, 8 μg filamentous haemagglutinin and 2.5 μg pertactin. The antigenic composition of FLU met the WHO recommendations for the preparation of the influenza vaccine for the 2006/2007 season (see table I)
Noninferiority between the concomitant and sequential administration of the two vaccines was demonstrated for both seroconversion and seroprotection rates against each of the three antigens contained in Fluarix® (see table VI;[49] the lower limit of the 95% CI for the between-group difference in seroprotection and seroconversion for each of the antigens contained in the Fluarix® vaccine was ≥−10%). Seroprotection rates (percentage of subjects with serum antibody levels ≥0.1 IU/mL) for diphtheria and tetanus were high (≥94.1%) for both vaccine regimens, and immune responses to these antigens with concomitant vaccine administration were noninferior to those when the vaccines were administered sequentially. The pertussis components of Tdap (pertussis toxoid, filamentous haemagglutinin and pertactin) were immunogenic in both groups, with increases in antibody geometic mean concentrations (GMCs) of at least 8-fold for all three antigens. Although antibody concentrations for pertussis toxoid antigens were numerically lower with concomitant than sequential vaccine administration, concomitant vaccine administration was noninferior to sequential administration with respect to anti-pertussis toxoid concentrations. For filamentous haemagglutinin and pertactin, the between-group differences in antibody concentrations did not fulfil the prespecified limits for noninferiority, as the lower limits of the 95% CI for the GMC ratios were marginally lower than the predefined noninferiority limits.
Vaccines against meningococcal disease and seasonal influenza can be coadministered without compromising the immunogenicity of either vaccine, according to data from a randomized, multicentre, phase III trial (data presented in an abstract).[50] Subjects aged 18–55 years were randomized to receive a candidate vaccine (meningococcal serogroups A, C, W-135, Y-tetanus toxoid conjugate vaccine [ACWY-TT] alone; n = 311), ACWY-TT coadministered with Fluarix® (n = 105) or a licensed meningococcal tetravalent polysaccharide vaccine alone (n = 104). ACWY-TT coadministered with Fluarix® met all criteria set out by the CHMP for all three influenza strains (see table III). Overall, >97% of subjects in this study (irrespective of vaccine group) achieved serum bactericidal antibody titres of >1 : 128 for all of the meningococcal serogroups A, C, W-135 and Y. Moreover, coadministration of ACWY-TT with Fluarix® was noninferior to administration of ACWY-TT alone with regards to the meningococcal serogroups A, W-135 and Y (the upper limit of the 95% CI of the serum bactericidal antibodies GMT ratio was <2.0 [1.8, 1.7 and 1.7, respectively]).
3. Protective Efficacy
3.1 Placebo-Controlled Trials
The protective efficacy of Fluarix® against culture-confirmed influenza A and/or B has been investigated in two randomized, double-blind, placebo-controlled, multicentre trials in adults aged 18–64 years (table VII).[23,31,32]
Protective efficacy of Fluarix® (FLU) in adults aged 18–64 years in randomized, double-blind, placebo (PL)-controlled, multicentre trials conducted in the Czech Republic[31,32] and Finland.[31] The antigenic composition of FLU met the WHO recommendations for the preparation of the influenza vaccine for the season in which it was administered (see table I). Subjects received a single 0.5 mL dose of the vaccine administered intramuscularly. Subjects were monitored for influenza-like illness from 2 weeks postvaccination until approximately 7 months later (until the end of April of the appropriate year). The intent-to-treat data are presented
In both these studies (see section 2.2.1 for immunogenicity data from these studies), the primary endpoint was the occurrence of culture-confirmed influenza (specified as that due to viral strains antigenically matched to the vaccine[31] or any influenza strain[32]). Influenza was defined as an episode of influenza-like illness occurring after the administration of the study vaccine for which a nasal or throat swab specimen yielded influenza virus A and/or B in cell culture.[31,32] The virus isolate was classified as matching the vaccine A and B strains based on its reaction with influenza strain-typing reagents. Influenza-like illness was a secondary endpoint and was defined as the presence of fever (oral temperature ≥37.8°C) plus cough and/or sore throat,[32] or presence of at least one systemic symptom (fever and/or myalgia) and at least one respiratory symptom (cough and/or sore throat).[31] Passive (reporting by subjects to the investigator) and active (biweekly phone contact) surveillance (from 2 weeks to 7 or 9 months after vaccination) was used to detect cases of influenza. In general, nasal and throat swab specimens were collected by day 2 after the onset of symptoms.
The first efficacy study was conducted during a low attack rate season (2005/2006) in which few subjects reported cases of influenza, with only 46 cases of culture-confirmed influenza being reported in the study cohort (10 influenza A and 36 influenza B).[32] The attack rate in this study in the placebo group was 0.9% (table VII); the study was powered on the assumption that the attack rate of culture-confirmed influenza in the placebo group would be 4%. Moreover, among the B isolates, 35 isolates were identified as being antigenically different (B/Hong Kong 330/2001-like [B/Victoria/2/87 lineage]) to the B strain (B/Yamagata/16/88 lineage) that was used to produce the vaccine for the 2005/2006 season. This study did not demonstrate the efficacy of Fluarix® vaccine (table VII), according to the pre-set criteria of this study. As noted by the study researchers, this study illustrates the challenges of conducting an influenza vaccine efficacy trial during a single season when variations in disease attack rates and the circulating strains cannot be predicted in advance.
However, the efficacy of Fluarix® was demonstrated in the subsequent season (2006/2007) in the other placebo-controlled study.[31] The vaccine efficacy was 66.9% (95% CI 51.9, 77.4; p < 0.001) against culture-confirmed influenza A and/or B due to strains antigenically matched to the vaccine. The vaccine efficacy against culture-confirmed influenza A and/or B due to any strain was 61.6% (95% CI 46.0, 72.8; p < 0.001). Most cases of culture-confirmed influenza were identified as H3N2 influenza (95%), with most cases (85%) being antigenically matched to the strain contained within the vaccine. This study used a less stringent case definition of influenza-like illness than the other placebo-controlled study and used a sample size that was based on a lower rate of viral attack (2% in the placebo group).
3.2 Pregnant Women and Infants
Fluarix® vaccination of women in the third trimester of pregnancy demonstrated efficacy in reducing the incidence of respiratory illnesses in these women, as well as reducing the rate of laboratory-confirmed influenza and respiratory illnesses in their new-born infants, according to data from a randomized, active-comparator trial conducted in Bangladesh (2004/2005).[51] Women were randomized to Fluarix® formulated for the 2004 Southern Hemisphere (n = 172) or a 23-valent pneumococcal polysaccharide vaccine (n = 168; control group) and then interviewed weekly (until 24 weeks after the birth) to assess illnesses. Women with febrile respiratory illnesses were assessed clinically and their infants tested for influenza antigens. The primary endpoint was the first episode of laboratory-confirmed influenza in infants aged <24 weeks. Mothers, families and study staff who collected data regarding illness and adverse events were blinded to the study-group assignment. Clinical efficacy was calculated according to the formula (1 — incident rate ratio) × 100.
Laboratory-confirmed influenza occurred in fewer infants whose mothers had been vaccinated with Fluarix®, compared with infants whose mothers received the control vaccine (6 vs 16 cases; vaccine efficacy of 63% [95% CI 5, 85.4]; p < 0.05).[51] Respiratory illness with fever occurred in 110 of the infants whose mothers had been vaccinated with Fluarix® and 153 of the infants whose mothers had received the control vaccine (p < 0.05), representing a vaccine efficacy of 29% (95% CI 7, 46). Fluarix®, compared with the control vaccine, was significantly more efficacious in preventing respiratory illness with fever in the mothers (50 vs 77 episodes; vaccine efficacy 36% [95% CI 4, 57]; p < 0.05).
4. Reactogenicity
This section reviews data relating to the reactogenicity of Fluarix® from the trials reviewed in sections 2 and 3, as well as relevant, contributory data from the US prescribing information[22] and the EU summary of product characteristics.[52] Generally, local and general adverse event data were solicited from the study participants for the day of vaccination and 3 days after. Unsolicited adverse events that occurred within 21 days after vaccination were recorded in standardized diaries. Grade 3 adverse events were defined as those that prevented normal activities, redness or swelling >50 mm, or fever >39°C.
4.1 Adults
Fluarix® was generally well tolerated in adults in the trials reported in sections 2 and 3, with most local and general adverse events being transient and mild to moderate in intensity.
4.1.1 Comparative Trials
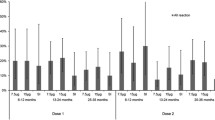
Solicited local and general adverse events that occurred in the large, randomized, double-blind, placebo-controlled trial in 952 evaluable subjects (reviewed in section 2.2.1)[30] and in the randomized, single-blind, active-comparator immunogenicity trial in 1827 evaluable subjects (section 2.2.2)[33] are shown in figure 4.[22,23] The most common adverse events in recipients of Fluarix® were pain, redness or swelling at the injection site, muscle aches, fatigue, headache and arthralgia.[22]
Solicited local and general adverse events with Fluarix® (FLU). Solicited adverse events were recorded during the 4-day period after vaccination with a single 0.5 mL intramuscular dose of FLU in (a) adults (aged 18–64 years) randomized to FLU (n = 760) or placebo (PL; n = 192) in a double-blind, multicentre study[23,30] and in (b) adults aged 18–64 years and those aged ≥65 years randomized to FLU (n = 917) or Fluzone® (FLZ; n = 910) in a large, single-blind trial.[23,33] * p < 0.05, ** p ≤ 0.001 vs PL.
In the placebo-controlled immunogenicity trial,[23,30] pain at the injection site (p < 0.001), redness (p = 0.016) and myalgia (p = 0.001) were the solicited adverse events that occurred more commonly in recipients of Fluarix® than placebo (figure 4).[23,30] Unsolicited adverse events that occurred in recipients of Fluarix® and placebo in this trial included upper respiratory tract infection (3.9% vs 2.6%), nasopharyngitis (2.5% vs 1.6%), nasal congestion (2.2% vs 2.1%), diarrhoea (1.6% vs 0%), influenza-like illness (1.6% vs 0.5%), vomiting (1.4% vs 0%) and dysmenorrhoea (1.3% vs 1%); none of the between-group differences were statistically significant.[23,30]
A similar unsolicited adverse event profile was obtained with Fluarix® in the active-comparator trial (figure 4).[23,33] Unsolicited events that occurred in ≥1% of recipients of Fluarix® or Fluzone® included headache (2.8% vs 2.3%), back pain (1.5% vs 0.4%), pain in the extremity (1.2% vs 0.7%), pharyngolaryngeal pain (1.2% vs 0.9%), cough (1.1% vs 0.9%), fatigue (1.1% vs 0.7%), nasopharyngitis (1.0% vs 1.3%), nausea (0.4% vs 1.0%), arthralgia (0.3% vs 1.0%) and injection-site pruritus (0.2% vs 1.0%).[23,33]
In a subset of subjects (n = 460) from a large (n = 7652), randomized, double-blind, placebo-controlled efficacy trial (reviewed in section 3.1),[22,31] at least one unsolicited event was reported in 24.3% of Fluarix® recipients and 22.6% of placebo recipients. Unsolicited adverse events occurring in ≥1% of Fluarix® recipients and at a rate numerically greater than with placebo were injection-site pain (5.2% vs 1.3%), dysmenorrhoea (1.3% vs 0.6%) and migraine (1.0% vs 0%).
4.1.2 Annual Registration Studies
Fluarix® was generally well tolerated in adults aged 18–60 years enrolled in the annual registration trials (1992–2009) [section 2.2.3].[23,27]
In more recent seasons (2004/2005 to 2009/2010), solicited local adverse events (any severity; see table VIII) occurred in 2–67% of Fluarix® recipients, with most adverse events being mild to moderate in severity. General solicited symptoms (any severity) occurring in Fluarix® recipients included myalgia (8–27%), fatigue (16–22%), headache (13–20%), sweating (8–10%), arthralgia (6–10%), shivering (2–9%) and fever (0–2%). Most adverse events were mild to moderate in intensity. Unsolicited adverse events occurred in 5–18% of Fluarix® recipients, and no serious adverse event considered to be related to the study medication was reported.
Reactogenicity of Fluarix® in adults (aged 18–60 years) and the elderly (aged ≥60 years) in annual noncomparative registration trials conducted in Germany.[23] Data are obtained from the manufacturer’s clinical database.[23] Solicited adverse events (regardless of severity) reported in the 4-day postvaccination period in recipients of Fluarix® are shown. The antigenic composition of the Fluarix® vaccine met the WHO recommendations for the preparation of the influenza vaccine for the season in which it was administered (see table I). Subjects received a single 0.5 mL dose of the vaccine administered intramuscularly
4.2 Elderly
Fluarix® was generally well tolerated in the elderly in the trials reported in sections 2 and 3, with most adverse local and general adverse events being mild to moderate in intensity. The profile of solicited and unsolicited local and general adverse events was generally similar to that reported in younger recipients of Fluarix® (section 4.1).
Solicited local and general adverse events (figure 4) occurred in 2–19% of a subgroup of elderly subjects aged ≥65 years immunized with Fluarix® who were enrolled in a randomized, single-blind, active-comparator immunogenicity trial (2005/2006 season; see section 2.2.2).[22,23,33] Pain, redness and swelling were the most commonly reported local solicited adverse events in recipients of Fluarix® and its comparator in the 4 days postvaccination. The profile of solicited general adverse events after Fluarix® administration was similar to that in younger recipients (aged 18–64 years) of the vaccine, although the incidence of solicited adverse events tended to be numerically lower in the elderly than in adults (figure 4).
Fluarix® was generally well tolerated in subjects aged ≥60 years enrolled in the annual registration trials (1992–2009).[23,27]
In the seasons 2004–2009,[22,23] solicited local adverse events (any severity; see table VIII) occurred in 0–37% of Fluarix® recipients aged ≥60 years, with the adverse event profile being similar to that in the younger age group (aged 18–60 years). General solicited adverse events (any severity) occurring in Fluarix® recipients included myalgia (2–12%), fatigue (2–14%), headache (2–14%), sweating (2–9%), arthralgia (0–14%), shivering (0–9%) and fever (0–2%). Most adverse events were mild to moderate in intensity. Unsolicited adverse events occurred in 0–10% of Fluarix® recipients, and no serious adverse event considered to be related to the study medication was reported.
4.3 At-Risk Populations
In the studies conducted in at-risk populations (section 2.4),[27,37,38,41] Fluarix® was generally well tolerated. Local solicited reactions occurred in 0–14% of recipients of Fluarix® and fever or other general solicited adverse events occurred in ≤10% of Fluarix® recipients.
4.4 Rare Adverse Events
Immediate and presumably allergic reactions have occurred rarely after the administration of influenza vaccine and these are generally considered to be the result of hypersensitivity to certain vaccine constituents (e.g. residual egg protein).[22] Fluarix® contains only residual amounts of egg protein.[22]
Although the 1976/1977 swine influenza vaccination was associated with an increased risk of Guillain-Barré syndrome (GBS),[53] evidence for a causal relationship between subsequent vaccines prepared from other influenza viruses has not been clearly established (see section 6 for further discussion of this topic).
5. Dosage and Administration
Each dose of Fluarix® has been formulated to contain 45 μg of haemagglutinin (15 μg of each of the haemagglutinins of the three virus strains that are expected by the WHO to be circulating in the community in the upcoming winter).[22,52]
In adults, Fluarix® is indicated for active immunization for the prevention of disease caused by influenza virus subtypes A and type B contained in the vaccine (US label)[22] or for the prophylaxis of influenza, especially in those who run an increased risk of associated complications (EU label).[52]
In adults, Fluarix® should be administered as a single 0.5 mL dose by intramuscular injection, preferably in the region of the deltoid muscle of the upper arm.[22]
Fluarix® is contraindicated in persons with known systemic hypersensitivity reactions to egg proteins (a vaccine component) or a life-threatening reaction to previous administration of any influenza vaccine (US label)[22] or in persons with hypersensitivity to the active substances, to any of the excipients, to residues, to egg and to chicken protein (EU label).[52] Immunization should be postponed in patients with febrile illness or acute infection.[52]
Fluarix® does not contain thimerosal and does not contain more than 0.05 μg ovalbumin per dose.[22] The vaccine may contain residues of the following substances: formaldehyde, gentamicin sulphate, hydrocortisone and sodium desoxycholate.
Local prescribing information should be consulted for other specific details of indications, warnings, contraindications and precautions.
6. Place of Fluarix® in the Prevention of Seasonal Influenza
The disease burden of influenza is significant. In 2005, the annual global attack rate from non-pandemic influenza infections was estimated to be 5–10% in adults (age not specified).[4,54] The WHO estimates (2009) that there are ≈1.2 billion people worldwide at high risk for severe influenza outcomes: 385 million elderly aged >65 years, 140 million infants, and 700 million children and adults with an underlying chronic health problem. In addition, the WHO estimates that there are 24 million healthcare workers who should be immunized so that the disease is not spread to patients at greatest risk.[55]
Influenza can result in absenteeism, disruption in work, decreased productivity and increased healthcare costs.[4,54] In the US, an estimated annual average of 41 000 deaths and >200 000 hospitalizations are attributable to influenza infections.[56] The incidence of mortality may be higher during pandemics of influenza; the 1918 H1N1 ‘Spanish flu’ accounted for 50–100 million deaths worldwide;[57] the 1957/1958 H2N2 ‘Asian flu’ accounted for more than 1 million deaths worldwide and the 1968/1970 H3N2 ‘Hong Kong flu’ accounted for >700 000 deaths worldwide.[58]
Vaccination with a seasonal influenza vaccine remains the cornerstone in the management of influenza, reducing the morbidity and mortality of this disease, especially in those most at risk.[3,11,54] Nonpharmacological interventions (e.g. advising frequent handwashing and improved respiratory hygiene) have also been used to prevent the spread of influenza.[59,60] Limited data also suggest that community-level respiratory disease mitigation strategies (e.g. closing schools, avoiding mass gatherings or using respiratory protection) may also reduce influenza virus transmission.[61,62] A number of antiviral drugs are also available and include the adamantanes (e.g. amantadine and rimantadine) that block the M2 viral protein channel and the newer neuraminidase inhibitors (e.g. oseltamivir and zanamivir).[63] These antiviral agents can reduce the severity and duration of symptoms, the time to return to work and, potentially, the use of antibacterials and relief medications. However, these agents are effective only against the influenza virus and a rapid diagnosis of the disease is required if these agents are to be used to treat infected individuals. Moreover, many influenza viruses are resistant to the adamantanes and there is also increasing resistance against the neuraminidase inhibitors.[64]
The WHO lists more than 30 manufacturers of influenza vaccine worldwide.[65] Both inactivated and live, attenuated influenza vaccines are available.[3,4,11,54] Inactivated influenza vaccines including whole virus vaccines, split virus vaccines and subunit vaccines are available.[4] In most countries, whole virus vaccines have been replaced by less reactogenic split virus and subunit vaccines. Current inactivated vaccines are trivalent, containing 15 μg haemagglutinin of each of two influenza A subtypes (H1N1 and H3N2) and 15 μg haemagglutinin from one influenza B strain. Split or subunit virion vaccines are prepared by transferring the haemagglutinin and neuraminidase genomic segments from a circulating wild-type virus into a well characterized, high-yield egg-adapted or cell culture-adapted influenza virus strain such as the A/Puerto Rico/8/34 (PR8) virus strain. Usually, the reassortant virus with the desired haemagglutinin and neuraminidase gene combination is then grown in the allantoic cavity of embryonated hen’s eggs. Some vaccines are produced using mammalian cell lines such as MDCK, PERC-6 or Vero cells.[66] In split virion vaccines, the virus has been disrupted by a detergent. Subunit virion vaccines are composed of surface antigens and have been further purified by removal of other viral components. Some current formulations of trivalent, inactivated influenza vaccines include adjuvants such as immune-stimulating complexes, MF59 adjuvant or virosomes in order to increase immunogenicity. Inactivated vaccines are usually administered intramuscularly.
Live attenuated influenza vaccines are also available.[11] Although these vaccines can be easily administered via the nasal route to induce a broad mucosal and systemic immune response, they are not indicated in children aged <2 years or adults aged ≥50 years and there is a risk that the influenza virus could be transmitted from the vaccinee to at-risk individuals such as those who are severely immunocompromised. The efficacy of the live attenuated influenza vaccine has been compared with that of trivalent inactivated influenza vaccines in a population-based cohort study of more than a million military personnel aged 17–49 years vaccinated during the seasons 2004/2005, 2005/2006 and 2006/2007.[67] The trivalent inactivated vaccine was associated with significantly fewer medical encounters related to pneumonia and influenza than with the intranasal live attenuated influenza vaccine in this annually immunized population.
Other methods for improving the efficacy of the influenza vaccine have also been investigated, including increasing the dosage and modifying the delivery method (e.g. intranasal/aerosol delivery, transdermal or sublingual delivery).[68,69] Alternative methods of producing the vaccines have been investigated (e.g. recombinant DNA-based vaccines, cell culture-derived vaccines and peptide epitope vaccines). Various influenza vaccines that use these methodologies are now approved for use.
Influenza vaccines prevent approximately 70–90% of culture-confirmed influenza illness in healthy adults in industrialized countries, provided there is a good match between the vaccine antigens and circulating virus(es).[4,11,70] Administration of the influenza vaccine to employees at the University of Minnesota aged 50–64 years was associated with a reduction in influenza-like illness (odds ratio 0.48; 95% CI 0.27, 0.86), as well as fewer days of illness, absenteeism and impaired work performance during the influenza season.[71] Administration of the influenza vaccine to elderly non-institutionalized subjects may reduce the number of hospitalizations by 25–39%, as well as reducing mortality by 39–75% during the influenza season.[4]
Guidelines of the WHO[4] and other international institutions and societies[11,54,72–75] recommend influenza vaccination for those at greatest risk, including the elderly (aged ≥65 years) and those people with specific underlying medical conditions (including pulmonary and cardiac disease, diabetes and immunosuppression). In 2003, the World Health Assembly adopted a resolution to increase influenza vaccination coverage of all people at high risk, with the aim of vaccinating at least 75% of the elderly population by 2010.[4] Based on scientific and public health evidence, the European Centre for Disease Prevention and Control recommends seasonal influenza vaccination of the elderly (those aged ≥65 years) and people with chronic medical conditions.[73] In the US, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) currently recommends influenza vaccination for a wide range of at-risk populations, including pregnant women, subjects aged ≥50 years of age and children aged 6–59 months.[72] More recently, the ACIP voted to expand the recommendation for annual influenza vaccination to include all people aged ≥6 months, with the expanded recommendation to take effect in the 2010/2011 influenza season.[76]
The changing nature of the influenza genome enables the virus to evade natural population immunity, but presents difficulties for the manufacturers of influenza vaccines.[4,15,16] Each season’s vaccine must closely match the antigenic composition of the viral strains of influenza in circulation that year if it is to maximize immunogenicity in its recipients. Thus, the WHO has established a worldwide surveillance system allowing identification and isolation of viral strains circulating the different parts of the globe. The WHO then makes recommendations on the composition of the influenza vaccine for the coming influenza season (in February for the Northern Hemisphere and in September for the Southern Hemisphere) depending on which strains are the greater threat and considered the most virulent. Based on the WHO recommendations, the influenza vaccines contain the predicted antigenic variants of influenza A (H3N2 and H1N1) and influenza B viruses (see tables I and II for those used in the production of Fluarix®).[20] During 2010, production capacity for trivalent influenza vaccine is estimated to have sharply increased to over 800 million doses.[77,78] Many months are needed for the manufacturing of the vaccine and its delivery to sites of vaccination. Although in most years the recommendations accurately predict a close antigenic match between the vaccine and epidemic virus strain circulation, in some years there has been a mismatch between the predominant circulating strain and that used to prepare the vaccine, with a consequent lack of vaccine effectiveness.[68,79,80] In particular, in recent years, there has been a mismatch between the influenza B virus strain recommended by WHO for inclusion in the seasonal vaccine and the B influenza virus strain in circulation during the season for which the vaccine was intended.[81,82]
The measurement of HI antibody response is important in the assessment of new influenza vaccines, with rates of seroprotection and seroconversion being commonly used. Although there is no HI antibody titre that will ensure protective immunity, regulatory authorities in the US and Europe have established immunogenicity criteria that must be met if the influenza vaccine is to be licensed (table III). These guidelines are based on the repeated demonstration that serum HI antibody titres correlate inversely with the frequency of influenza illnesses in those who have been vaccinated.[68] Nevertheless, there are a number of variables that affect antibody responses including the vaccine formulation, the age of the subjects, the immunocompetence of the subjects and the number of vaccines previously received. Responses to vaccination in older adults are generally lower than those in younger adults.[83]
The majority of healthy adults and elderly have high titres of HI antibody after vaccination, with Fluarix® exceeding the immunogenicity criteria that made it acceptable for licensure in the US and Europe (section 2). In a large, placebo-controlled, double-blind trial in subjects aged 18–64 years in the US (2004/2005), postvaccination seroconversion rates against the H1N1, H3N2 and B antigens were 60–78% and postvaccination seroprotection rates were 97–99% after Fluarix® administration. Another phase III trial conducted in the US (2005/2006) established the noninferiority of Fluarix® versus another trivalent inactivated influenza virus vaccine in subjects aged ≥18 years, including a subgroup of elderly subjects (section 2.2.2). Although the immune response in the elderly was generally lower than that in adults, in annual registration trials conducted in Europe, Fluarix® consistently exceeds all the immunogenicity criteria set by the EU CHMP for both groups (sections 2.2.3 and 2.3.2). Data from randomized studies indicated that Fluarix® may be administered together with other vaccines (e.g. Tdap and a meningococcal ACWY vaccine) without compromising the immunogenicity of either vaccine (section 2.7).
Cell-mediated immunity is also important in controlling respiratory viruses in the lungs.[84] Vaccines that enhance T-cell immunity in addition to robust antibody responses are likely to be the most protective. Data investigating cell-mediated responses in recipients of influenza vaccines (including Fluarix®) are limited.[85] Nevertheless, it is evident that annual immunization has some impact on both systemic and mucosal T-cell responses (section 2.5) and further investigation in this area is warranted.
Fluarix® provided seroprotective titres against homologous drifts of influenza A/H1N1 and influenza B, although the protection against subsequent drift variants of influenza A/H3N2 was more variable, according to data from a serological study (section 2.6). Data from two small studies that have investigated the seroprotective titres of Fluarix® against H5N1 have been inconsistent.
Randomized controlled trials that measure laboratory-confirmed influenza virus infection demonstrate the efficacy of trivalent inactivated influenza vaccines.[11] When the vaccine and circulating viruses are antigenically similar, trivalent influenza vaccines prevent 70–90% of laboratory-confirmed influenza illness in subjects aged <64 years.[11] Nevertheless, efficacy estimates of the influenza vaccines depend upon various factors such as the degree of antigenic match between the vaccine and circulating virus strains, the virulence of the circulating virus, the clinical case definition of the influenza-like illness used and the surveillance methodology used. For example, in a season in which the attack rate was very low and when the circulating viruses were not antigenically matched to those used to produce the Fluarix® vaccine, a randomized, placebo-controlled trial did not demonstrate the efficacy of the vaccine (section 3). However, in a subsequent season when the vaccine was well matched to the circulating strain, the vaccine efficacy of Fluarix® was 66.9% against culture-confirmed influenza A and/or B due to strains antigenically matched to the vaccine in a placebo-controlled study (section 3). The sample size of this study was based on the assumption of a lower attack rate, and this study employed a less stringent case definition of influenza-like illness than the study in the previous season. An analysis of whether the immune response elicited by a vaccine correlates with protection against influenza illness will depend upon the specific case definition used. Given the case definition can vary between trials, this further adds to the challenge of comparing outcomes between efficacy trials.
The burden of influenza morbidity and mortality is generally greatest amongst the elderly, and therefore the efficacy of the influenza vaccine within this population is of particular relevance.[11,70,86] An early randomized trial conducted in 1991 in subjects aged ≥60 years with no substantial co-morbid conditions demonstrated that recipients of the trivalent inactivated influenza vaccine had a 58% lower risk of developing laboratory-confirmed influenza than those administered placebo.[87] However, as annual vaccination of the elderly has become widely recommended, no further placebo-controlled trials to determine the efficacy of the influenza vaccine have been conducted due to ethical considerations. Instead, subsequent observational cohort studies have sought to demonstrate the effectiveness of the influenza vaccine in this population, including in those living in the community[88,89] and those living in nursing homes.[90,91] For example, a cohort study of community-dwelling elderly aged >65 years (involving 713 872 person-seasons of observation) found that vaccination against influenza with a trivalent inactivated vaccine was associated with a 27% reduction in the risk of hospitalization for pneumonia or influenza and a 48% reduction in the risk of death.[88] However, questions have been raised over the potential bias of the observational studies.[92–94]
A recent review of the literature concluded that the role of vaccines for preventing influenza in the elderly still needed clarification, and the authors of the review suggest that to resolve this uncertainty, an adequately powered, publicly funded, placebo-controlled trial running over several seasons needs to be conducted.[94] Further investigation of the role of influenza vaccination in the elderly is certainly warranted, with future areas of clinical investigation also likely to include the adjuvantation of vaccines.[34] In the interim, the decision to offer influenza vaccination to elderly or at-risk groups can be seen as a reasonable and safe policy.[86]
Although influenza vaccines are recommended for other high-risk patients (such as immunocompromised patients, or pregnant women, or children [beyond the scope of this review]),[4,11,54,72–75] well designed trials investigating the immunogenicity of influenza vaccines in these groups are limited.[95] Data from small, open-label trials have demonstrated the immunogenicity of Fluarix® in a number of at-risk groups (including the immunocompromised; see section 2.4). In a well controlled trial in pregnant women in Bangladesh, Fluarix® demonstrated efficacy (36%) in preventing the rate of respiratory illness with fever in the mother, with an efficacy of 63% in preventing laboratory-confirmed influenza in the infants (aged up to 6 months) [section 3.2].
Fluarix® was generally well tolerated in adults and the elderly in well designed clinical trials and in the annual European registration trials, with most local and general adverse events being transient and mild to moderate in intensity. The most common adverse events in recipients of Fluarix® were pain, redness or swelling at the injection site, muscle aches, fatigue, headache and arthralgia (section 4). In the studies conducted in at-risk populations, Fluarix® was generally well tolerated.
Although data indicate that vaccination of asthmatic adults with a trivalent inactivated split-virus influenza vaccine is generally well tolerated and the risk of pulmonary complications is small,[96] studies specifically investigating the reactogenicity of Fluarix® in asthmatic adults have not been conducted.
An increased risk of GBS was associated with the 1976/1977 swine influenza vaccination, with a rate of one case per 100 000 subjects vaccinated.[53] However, in subsequent studies, influenza vaccination has been associated with little or no increased risk of this syndrome.[11,97–99] One thousand cases of GBS were reported to the US Vaccine Adverse Event Reporting System (VAERS) following trivalent inactivated influenza vaccination of adults from 1990 to 2005, with the onset of the syndrome being within 6 weeks in 774 cases.[98] Death or disability as a result of GBS after influenza vaccination occurred in about one-fifth of affected subjects; a rate that is similar to that in the general population after GBS occurrence.[98] Another study (that also used VAERS data from the same period) estimated the rate of GBS following influenza vaccination was 0.78 cases per million vaccinations (including serious and nonserious reports).[99] A recent estimate of the overall incidence of GBS in the general population (derived largely from studies in the US and Europe) was between 1.1 and 1.4/100 000/year.[100]
Fluarix® is highly immunogenic and well tolerated in healthy adults and the elderly, and exceeds the criteria that make it acceptable for licensure in the US and Europe. Moreover, in a year when the vaccine was well matched to the circulating strain, Fluarix® demonstrated efficacy against culture-confirmed influenza A and/or B due to strains antigenically matched to the vaccine. Fluarix® demonstrated efficacy in reducing the rate of laboratory-confirmed influenza and respiratory illnesses in pregnant women and their new-born infants in a randomized trial. In conclusion, Fluarix® is an important means of decreasing the impact of seasonal influenza viruses on adults and the elderly.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Isabel Leroux-Roels received honoraria and travel grants from GlaxoSmithKline Biologicals. She assisted in the conduct of clinical studies of seasonal influenza vaccines for GlaxoSmithKline Biologicals.
References
Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clin Infect Dis 2003 Oct 15; 37(8): 1094–101
U.S. Department of Health and Human Services. Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines [online]. Available from URL: http://www.fda.gov/cber/guidelines.htm [Accessed 2010 Mar 24]
Stephenson I, Nicholson KG. Influenza: vaccination and treatment. Eur Respir J 2001 Jun; 17(6): 1282–93
WHO. WHO position paper: influenza vaccines [online]. Available from URL: http://www.who.int/wer/2005/wer8033/en/index.html [Accessed 2010 Apr 14]
Glezen WP, Greenberg SB, Atmar RL, et al. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000 Jan 26; 283(4): 499–505
Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004 Sep 15; 292(11): 1333–40
Kandel R, Hartshorn KL. Prophylaxis and treatment of influenza virus infection. Biodrugs 2001; 15(5): 303–23
Tosh PK, Jacobson RM, Poland GA. Influenza vaccines: from surveillance through production to protection. Mayo Clin Proc 2010 Mar; 85(3): 257–73
Webster RG, Bean WJ, Gorman OT, et al. Evolution and ecology of influenza A viruses. Microbiol Rev 1992 Mar; 56(1): 152–79
Chen J, Deng YM. Influenza virus antigenic variation, host antibody production and new approach to control epidemics. Virol J 2009; 6: 30
Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009 Jul 31; 58(RR-8): 1–52
GlaxoSmithKline. Fluarix™: product monograph. Rixensart, Belgium: GlaxoSmithKline Biologicals, 2008 Dec
Ghedin E, Sengamalay NA, Shumway M, et al. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature 2005 Oct 20; 437(7062): 1162–6
Nakajima S, Nobusawa E, Nakajima K. Variation in response among individuals to antigenic sites on the HA protein of human influenza virus may be responsible for the emergence of drift strains in the human population. Virology 2000 Aug 15; 274(1): 220–31
Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine 2007 Sep 28; 25(39–40): 6852–62
Treanor J. Influenza vaccine: outmaneuvering antigenic shift and drift. N Engl J Med 2004 Jan 15; 350(3): 218–20
Poland GA, Jacobson RM, Targonski PV. Avian and pandemic influenza: an overview. Vaccine 2007 Apr 20; 25(16): 3057–61
Belshe RB. The origins of pandemic influenza: lessons from the 1918 virus. N Engl J Med 2005 Nov 24; 353(21): 2209–11
WHO. Pandemic influenza preparedness; WHO guidance document (2009) [online]. Available from URL: http://www.who.int/csr/disease/influenza/PIPGuidance09.pdf [Accessed 2010 May 13]
WHO. Recommendations for influenza vaccines [online]. Available from URL: http://www.who.int/csr/disease/influenza/vaccinerecommendations/en/ [Accessed 2010 Apr 14]
WHO. Recommendations for influenza vaccine composition [online]. Available from URL: http://www.who.int/csr/disease/influenza/vaccinerecommendations1/en/index.html [Accessed 2010 May 11]
GlaxoSmithKline. Fluarix (influenza virus vaccine): suspension for intramuscular injection [online]. Available from URL: http://us.gsk.com/products/assets/us_fluarix.pdf [Accessed 2010 Mar 28]
GlaxoSmithKline. GSK Clinical Trial Registry [online]. Available from URL: http://www.gsk-clinicalstudyregister.com [Accessed 2010 May 11]
European Committee for Medicinal Products for Human Use. Note for guidance on harmonization of requirements for influenza vaccines (CPMP/BWP/214/96) [online]. Available from URL: http://www.ema.europa.eu/pdfs/human/bwp/021496en.pdf [Accessed 2010 Mar 24]
Künzel W, Engelmann H, D’Hondt E. Immune response to influenza vaccination [letter]. Lancet 1994 Jan 15; 343(8890): 173
Künzel W, Glathe H, Engelmann H, et al. Kinetics of humoral antibody response to trivalent inactivated split influenza vaccine in subjects previously vaccinated or vaccinated for the first time. Vaccine 1996 Aug; 14(12): 1108–10
Hehme NW, Künzel W, Petschke F, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Investig 2002; 22(11): 751–69
Saenger R, Kuenzel W, Tuerk G, et al. Influenza split vaccine: how long will it protect? [abstract plus poster]. Tenth International Congress on Infectious Diseases; 2002 Mar 11–14; Singapore
Hobson D, Curry RL, Beare AS, et al. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972 Dec; 70(4): 767–77
Treanor JJ, Campbell JD, Brady RC, et al. Rapid licensure of a new, inactivated influenza vaccine in the United States. Hum Vaccin 2005 Nov 31; 1(6): 239–44
Beran J, Vesikari T, Wertzova V, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis 2009 Dec 15; 200(12): 1861–9
Beran J, Wertzova V, Honegr K, et al. Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis 2009; 9: 2
Campbell JD, Chambers C, Brady R, et al. A phase III study to evaluate the immunogenicity and safety of Fluarix® compared with Fluzone® in adults in the U.S [abstract and poster]. Tenth Annual Conference on Vaccine Research; 2007 Apr 30-May 2; Baltimore (MD)
Baras B, Bouveret N, Devaster JM, et al. A vaccine manufacturer’s approach to address medical needs related to seasonal and pandemic influenza viruses. Influenza Other Respi Viruses 2008 Nov; 2(6): 251–60
Ruf BR, Colberg K, Frick M, et al. Open, randomized study to compare the immunogenicity and reactogenicity of an influenza split vaccine with an MF59-adjuvanted subunit vaccine and a virosome-based subunit vaccine in elderly. Infection 2004 Aug; 32(4): 191–8
Van Hoecke Ch, Prikazsky V, Uto I. Immunogenicity of an inactivated split influenza vaccine in institutionalized elderly patients. Gerontology 1996 Jul; 42(4): 190–8
Jo YM, Song JY, Hwang IS, et al. Dose sparing strategy with intradermal influenza vaccination in patients with solid cancer. J Med Virol 2009 Apr; 81(4): 722–7
Burbach G, Bienzle U, Stark K, et al. Influenza vaccination in liver transplant recipients. Transplantation 1999 Mar 15; 67(5): 753–5
Brydak LB, Machala M, Laguna P, et al. Antibody response to influenza vaccination in splenectomized patients in Poland. J Clin Immunol 2004; 24(3): 225–36
Brydak LB, Calbecka M. Immunogenicity of influenza vaccine in patients with hemato-oncological disorders. Leuk Lymphoma 1999; 32(3-4): 369–74
Del Porto F, Lagana B, Biselli R, et al. Influenza vaccine administration in patients with systemic lupus erythe-matosus and rheumatoid arthritis: safety and immunogenicity. Vaccine 2006 Apr 12; 24(16): 3217–23
Wang S, Lei H, Wang J, et al. The cellular expression in healthy subjects before and after influenza vaccination [abstract no. G1-1201]. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008 Oct 25–28; Washington, DC
Brokstad KA, Eriksson JC, Cox RJ, et al. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis 2002 Apr 1; 185(7): 878–84
Guthrie T, Hobbs CG, Davenport V, et al. Parenteral influenza vaccination influences mucosal and systemic T cell-mediated immunity in healthy adults. J Infect Dis 2004 Dec 1; 190(11): 1927–35
Brokstad KA, Cox RJ, Eriksson JC, et al. High prevalence of influenza specific antibody secreting cells in nasal mucosa. Scand J Immunol 2001 Jul-2001 31; 54(1–2): 243–7
Gioia C, Castilletti C, Tempestilli M, et al. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg Infect Dis 2008 Jan; 14(1): 121–8
Tang JW, Ngai KL, Chan PK. Lack of cross-immune reactivity against influenza H5N1 from seasonal influenza vaccine in humans. J Med Virol 2008 Nov; 80(11): 1992–6
Heckler R, Billot A, Engelmann H. Cross-protection against homologous drift variants of influenza A and B after vaccination with split vaccine. Intervirology 2007; 50(1): 58–62
Weston WM, Chandrashekar V, Friedland LR, et al. Safety and immunogenicity of a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine when co-administered with influenza vaccine in adults. Hum Vaccin 2009 Dec 31; 5(12): 866–58
Macalalad N, Aplasca-De Los Reyes MR, Dimaano E. The candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate vaccine (MenACWY-TT) and the seasonal influenza virus vaccine are immunogenic and well-tolerated when coadministered in adults [abstract]. Third Northern European Conference on Travel Medicine; 2010 May 26–29; Hamburg
Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants [published erratum appears in N Engl J Med 2009 Feb 5; 360 (6): 648]. N Engl J Med 2008 Oct 9; 359(15): 1555–64
GlaxoSmithKline UK. Summary of product characteristics: Fluarix® [online]. Available from URL: http://www.medicines.org.uk/emc/medicine/2038/SPC/Fluarix [Accessed 2010 Jun 3]
Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol 1979 Aug; 110(2): 105–23
Harper SA, Bradley JS, Englund JA, et al. Seasonal influenza in adults and children-diagnosis, treatment, chemopro-phylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009 Apr 15; 48(8): 1003–32
WHO. Acute respiratory infections (update September 2009) [online]. Available from URL: http://www.who.int/vaccine_research/diseases/ari/en/index1.html [Accessed 2010 Apr 26]
Dushoff J, Plotkin JB, Viboud C, et al. Mortality due to influenza in the United States: an annualized regression approach using multiple-cause mortality data. Am J Epidemiol 2006 Jan 15; 163(2): 181–7
Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med 2002 Spring; 76(1): 105–15
Rajagopal S, Treanor J. Pandemic (avian) influenza. Semin Respir Crit Care Med 2007 Apr; 28(2): 159–70
Grayson ML, Melvani S, Druce J, et al. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis 2009 Feb 1; 48(3): 285–91
Jefferson T, Foxlee R, Del Mar C, et al. Interventions for the interruption or reduction of the spread of respiratory viruses. Cochrane Database Syst Rev 2007; (4): CD006207
Bell DM. Non-pharmaceutical interventions for pandemic influenza, international measures. Emerg Infect Dis 2006 Jan; 12(1): 81–7
Inglesby TV, Nuzzo JB, O’Toole T, et al. Disease mitigation measures in the control of pandemic influenza. Biosecur Bioterror 2006; 4(4): 366–75
Lynch 3rd JP, Walsh EE. Influenza: evolving strategies in treatment and prevention. Semin Respir Crit Care Med 2007 Apr; 28(2): 144–58
Smith JR, Ariano RE, Toovey S. The use of antiviral agents for the management of severe influenza. Crit Care Med 2010 Apr; 38 (4 Suppl.): e43–51
WHO. Influenza vaccine manufacturers [online]. Available from URL: http://www.who.int/csr/disease/influenza/Influenza_vaccine_manufacturers2009_05.pdf [Accessed 2010 Apr 27]
Audsley JM, Tannock GA. Cell-based influenza vaccines: progress to date. Drugs 2008; 68(11): 1483–91
Wang Z, Tobler S, Roayaei J, et al. Live attenuated or inactivated influenza vaccines and medical encounters for respiratory illnesses among US military personnel. JAMA 2009 Mar 4; 301(9): 945–53
Couch RB. Seasonal inactivated influenza virus vaccines. Vaccine 2008 Sep 12; 26 Suppl. 4: D5–9
Tosh PK, Poland GA. Emerging vaccines for influenza. Expert Opin Emerg Drugs 2008 Mar; 13(1): 21–40
Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine 2008 Sep 12; 26 Suppl. 4: D17–22
Nicol KL, D’Heilly SJ, Greenberg ME, et al. Burden of influenza-like illness and effectiveness of influenza vaccination among working adults aged 50–64 years. Clin Infect Dis 2009 Feb 1; 48(3): 292–8
Centers for Disease Control and Prevention. Seasonal influenza 2009–2010: ACIP vaccination recommendations [online]. Available from URL: http://www.cdc.gov/flu [Accessed 2010 Apr 14]
European Centre for Disease Prevention and Control. Guidance: priority risk groups for influenza vaccination [online]. Available from URL: http://ecdc.europa.eu/en/publications/Publications/0808_GUI_Priority_Risk_Groups_for_Influenza_Vaccination.pdf [Accessed 2010 Apr 15]
National Advisory Committee on Immunization. Statement on seasonal trivalent inactivated influenza vaccine (TIV) for 2009–2010. Can Commun Dis Rep 2009 Oct; 35 (ASC-6): 1–41
The Council of the European Union. Council recommendation of the 22 December 2009 on seasonal influenza vaccination [online]. Available from URL: http://www.epha.org/IMG/pdf/Council_Reccomendation_on_seasonal_flu_vaccine.pdf [Accessed 2009 Apr 15]
Centres for Disease Control and Prevention. CDC’s Advisory Committee on Immunization Practices (ACIP) recommends universal annual influenza vaccination [online]. Available from URL: http://www.cdc.gov/media/pressrel/2010/r100224.htm [Accessed 2010 Apr 14]
Partridge J, Kieny MP, The World Health Organization H1N1 influenza vaccine Task Force. Global production of seasonal and pandemic (H1N1) influenza vaccines in 2009–2010 and comparison with previous estimates and global action plan targets. Vaccine 2010; 28(30): 4709–12
Collin N, de Radigues X. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine 2009 Aug 20; 27(38): 5184–6
Skowronski DM, Masaro C, Kwindt TL, et al. Estimating vaccine effectiveness against laboratory-confirmed influenza using a sentinel physician network: results from the 2005–2006 season of dual A and B vaccine mismatch in Canada. Vaccine 2007 Apr 12; 25(15): 2842–51
Legrand J, Vergu E, Flahault A. Real-time monitoring of the influenza vaccine field effectiveness. Vaccine 2006 Nov 10; 24(44-46): 6605–11
Richard SA, Viboud C, Miller MA. Evaluation of Southern Hemisphere influenza vaccine recommendations. Vaccine 2010 Mar 24; 28(15): 2693–9
Barr IG, McCauley J, Cox N, et al. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 Northern Hemisphere season. Vaccine 2010 Feb 3; 28(5): 1156–67
Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006 Feb 20; 24(8): 1159–69
Doherty PC, Topham DJ, Tripp RA, et al. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev 1997 Oct; 159: 105–17
McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol 2006 May 15; 176(10): 6333–9
Knottnerus JA. Influenza vaccination in the elderly: current evidence and uncertainties. J Clin Epidemiol 2009 Jul; 62(7): 675–6
Govaert TM, Thijs CT, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. JAMA 1994 Dec 7; 272(21): 1661–5
Nichol KL, Nordin JD, Nelson DB, et al. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med 2007 Oct 4; 357(14): 1373–81
Vu T, Farish S, Jenkins M, et al. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine 2002 Mar 15; 20(13-14): 1831–6
Monto AS, Hornbuckle K, Ohmit SE. Influenza vaccine effectiveness among elderly nursing home residents: a cohort study. Am J Epidemiol 2001 Jul 15; 154(2): 155–60
Arden N, Monto AS, Ohmit SE. Vaccine use and the risk of outbreaks in a sample of nursing homes during an influenza epidemic. Am J Public Health 1995 Mar; 85(3): 399–401
Glezen WP, Simonsen L. Commentary: benefits of influenza vaccine in US elderly. New studies raise questions. Int J Epidemiol 2006 Apr; 35(2): 352–3
Jefferson T. Influenza vaccination: policy versus evidence. BMJ 2006 Oct 28; 333(7574): 912–5
Jefferson T, Di Pietrantonj C, Al-Ansary LA, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev 2010; (2): CD004876
Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis 2006 Nov 1; 194 Suppl. 2: S133–8
Nicholson KG, Nguyen-Van-Tam JS, Ahmed AH, et al. Randomised placebo-controlled crossover trial on effect of inactivated influenza vaccine on pulmonary function in asthma. Lancet 1998 Jan 31; 351(9099): 326–31
Haber P, Sejvar J, Mikaeloff Y, et al. Vaccines and Guillain-Barre syndrome. Drug Saf 2009; 32(4): 309–23
Souayah N, Nasar A, Suri MF, et al. Guillain-Barre syndrome after vaccination in United States: data from the Centers for Disease Control and Prevention/Food and Drug Administration Vaccine Adverse Event Reporting System (1990–2005). J Clin Neuromuscul Dis 2009 Sep; 11(1): 1–6
Vellozzi C, Burwen DR, Dobardzic A, et al. Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine 2009 Mar 26; 27(15): 2077–83
McGrogan A, Madle GC, Seaman HE, et al. The epidemiology of Guillain-Barre syndrome worldwide: a systematic literature review. Neuroepidemiology 2009; 32(2): 150–63
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J. Beran, Department of Infectious Diseases, University Hospital, Hradec, Králové, Czech Republic; H. Bhally, Waitemata District Health Board, Auckland, New Zealand; P. Chang, Department of Microbiology, The Chinese University of Hong Kong, Hong Kong; D.W.-S. Chu, Family Medicine and Primary Healthcare, Family Medicine Training Centre, Wanchai, Hong Kong; R. Saenger, Rostock, Germany.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘influenza virus vaccine’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘influenza virus vaccine’ and (‘split virion’ or ‘inactivated’ or ‘egg-derived’). Searches were last updated 8 July 2010.
Selection: Studies in adult and elderly subjects who received Fluarix®. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant immunogenicity data are also included.
Index terms: Influenza-virus vaccine, immunogenicity, reactogenicity, tolerability.
Rights and permissions
About this article
Cite this article
Curran, M.P., Leroux-Roels, I. Inactivated Split-Virion Seasonal Influenza Vaccine (Fluarix®). Drugs 70, 1519–1543 (2010). https://doi.org/10.2165/11205020-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11205020-000000000-00000