Abstract
Background
Considerable variability in sensitivity to corticosteroids (CS) has been observed among individuals with regard to both the natural and synthetic compounds. The role of genetic polymorphisms in modulating CS function, and hence in disease susceptibility, has been extensively analyzed. Their impact on therapeutic response still remains to be explored. The role of cytochrome P450 (CYP) 3A4 in corticosteroid metabolism, and that of the glucocorticoid receptor (NR3C1) in regulation of responsive genes, renders CYP3A4 and NR3C1 polymorphisms as potential candidates for pharmacogenetic analysis.
Aim
The aim of the study was to analyze the role of these polymorphisms in the outcome of a disease treated with CS drugs.
Methods
Towards this aim we analyzed the CYP3A4–290A/G substitution and three NR3C1 polymorphisms (200G/A, 1220A/G and BclI RFLP) in 222 children with acute lymphoblastic leukemia (ALL) whose treatment protocols, among other components, contained corticosteroid drugs.
Results
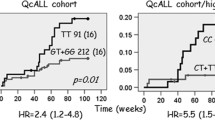
The analysis of survival probabilities in relation to the indicated genotypes showed only an association between homozygosity for allele G of the NR3C1 BclI RFLP polymorphism and overall survival (univariate and multivariate hazard ratio [HR] 2.7, 95% confidence interval [CI] 1.0, 7.6 and 5.2, 95% CI 1.4, 18.9, respectively). The association reflects a correlation with disease progression and prognosis, and may vary depending on risk of relapse.
Conclusion
A reduction in survival probability in children with ALL was associated with homozygosity for G allele of the NR3C1 BclI RFLP polymorphism, particularly in certain patient subgroups. Further analysis is required to replicate this finding and to understand the mechanism underlying the observed association.






Similar content being viewed by others
References
Bray PJ, Cotton RG. Variations of the human glucocorticoid receptor gene (NR3C1): pathological and in vitro mutations and polymorphisms. Hum Mutat 2003; 21: 557–68
Iida S, Nakamura Y, Fujii H, et al. A patient with hypocortisolism and Cushing’s syndrome-like manifestations: cortisol hyperreactive syndrome. J Clin Endocrinol Metab 1990; 70: 729–37
Lamberts SW, Koper JW, Biemond P, et al. Cortisol receptor resistance: the variability of its clinical presentation and response to treatment. J Clin Endocrinol Metab 1992; 74: 313–21
Pui CH. Acute lymphoblastic leukemia in children. Curr Opin Oncol 2000; 12: 3–12
Krynetski EY, Evans WE. Genetic polymorphism of thiopurine S-methyltransferase: molecular mechanisms and clinical importance. Pharmacology 2000; 61: 136–46
Krajinovic M, Labuda D, Sinnett D. Pharmacogenetics of childhood acute lymphoblastic leukemia. Curr Pharmacogenetics 2003; 1: 87–100
Holleman A, Cheok MH, den Boer ML, et al. Gene expression patterns in drug-resistant ALL cells and response to treatment. N Engl J Med 2004; 351: 533–42
Gaynon PS, Carrel AL. Glucocorticosteroid therapy in childhood acute lymphoblastic leukemia. Adv Exp Med Biol 1999; 457: 593–605
Kofler R. The molecular basis of glucocorticoid-induced apoptosis of lymphoblastic leukemia cells. Histochem Cell Biol 2000; 114: 1–7
Siebe H, Baude G, Lichtenstein I, et al. Metabolism of dexamethasone: sites and activity in mammalian tissues. Ren Physiol Biochem 1993; 16: 79–88
Chabner BA, Allegra CA, Curt GA, et al. Antineoplastic agents. In: Hardman JG, Gilman AG, Limbird LE, editors. The pharmacological basis of therapeutics. New York: McGraw-Hill, 1996
Hurwitz CA, Silverman LB, Schorin MA, et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer 2000; 88: 1964–9
Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs. In: Hardman JG, Limbird LE, Gilman AG, editors. The pharmacological basis of therapeutics. New York: McGraw-Hill, 1996: 1649–78
Feiner EI, Thompson MT, Ratliff AF, et al. Time course of recovery of adrenal function in children treated for leukemia. J Pediatr 2000; 137: 21–4
Waber DP, Carpentieri SC, Klar N, et al. Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J Pediatr Hematol Oncol 2000; 22: 206–13
Mattano LA, Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol 2000; 18: 3262–72
Greenstein S, Ghias K, Krett NL, et al. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res 2002; 8: 1681–94
Arai K, Chrousos GP. Hormone-nuclear receptor interactions in health and disease: glucocorticoid resistance. Baillieres Clin Endocrinol Metab 1994; 8: 317–31
Panarelli M, Fraser R. The glucocorticoid receptor and hypertension. Endocr Res 1994; 20: 101–16
de Lange P, Segeren CM, Koper JW, et al. Expression in hematological malignancies of a glucocorticoid receptor splice variant that augments glucocorticoid receptor-mediated effects in transfected cells. Cancer Res 2001; 61: 3937–41
Krett NL, Pillay S, Moalli PA, et al. A variant glucocorticoid receptor messenger RNA is expressed in multiple myeloma patients. Cancer Res 1995; 55: 2727–9
Longui CA, Vottero A, Adamson PC, et al. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm Metab Res 2000; 32: 401–6
Fleury I, Beaulieu P, Primeau M, et al. Characterization of the BclI polymorphism in the glucocorticoid receptor gene. Clin Chem 2003; 49: 1528–31
Rosmond R, Chagnon YC, Holm G, et al. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res 2000; 8: 211–8
Panarelli M, Holloway CD, Fraser R, et al. Glucocorticoid receptor polymorphism, skin vasoconstriction, and other metabolic intermediate phenotypes in normal human subjects. J Clin Endocrinol Metab 1998; 83: 1846–52
Ukkola O, Perusse L, Chagnon YC, et al. Interactions among the glucocorticoid receptor, lipoprotein lipase and adrenergic receptor genes and abdominal fat in the Quebec Family Study. Int J Obes Relat Metab Disord 2001; 25: 1332–9
Buemann B, Vohl MC, Chagnon M, et al. Abdominal visceral fat is associated with a BclI restriction fragment length polymorphism at the glucocorticoid receptor gene locus. Obes Res 1997; 5: 186–92
Weaver JU, Hitman GA, Kopelman PG. An association between a BclI restriction fragment length polymorphism of the glucocorticoid receptor locus and hyperinsulinaemia in obese women. J Mol Endocrinol 1992; 9: 295–300
Koper JW, Stolk RP, de Lange P, et al. Lack of association between five polymorphisms in the human glucocorticoid receptor gene and glucocorticoid resistance. Hum Genet 1997; 99: 663–8
van Rossum EF, Koper JW, Huizenga NA, et al. A polymorphism in the glucocorticoid receptor gene, which decreases sensitivity to glucocorticoids in vivo, is associated with low insulin and cholesterol levels. Diabetes 2002; 51: 3128–34
DeRijk RH, Schaaf M, de Kloet ER. Glucocorticoid receptor variants: clinical implications. J Steroid Biochem Mol Biol 2002; 81: 103–22
Huizenga NA, Koper JW, De Lange P, et al. A polymorphism in the glucocorticoid receptor gene may be associated with and increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab 1998; 83: 144–51
Abel SM, Maggs JL, Back DJ, et al. Cortisol metabolism by human liver in vitro: I. Metabolite identification and inter-individual variability. J Steroid Biochem Mol Biol 1992; 43: 713–9
Tomlinson ES, Lewis DF, Maggs JL, et al. In vitro metabolism of dexamethasone (DEX) in human liver and kidney: the involvement of CYP3A4 and CYP17 (17,20 LYASE) and molecular modelling studies. Biochem Pharmacol 1997; 54: 605–11
Garg V, Jusko WJ. Simultaneous analysis of prednisone, prednisolone and their major hydroxylated metabolites in urine by high-performance liquid chromatography. J Chromatogr 1991; 567: 39–47
Gentile DM, Tomlinson ES, Maggs JL, et al. Dexamethasone metabolism by human liver in vitro: metabolite identification and inhibition of 6-hydroxylation. J Pharmacol Exp Ther 1996; 277: 105–12
Rebbeck TR, Jaffe JM, Walker AH, et al. Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst 1998; 90: 1225–9
Amirimani B, Walker AH, Weber BL, et al. RESPONSE: re: modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst 1999; 91: 1588–90
Felix CA, Walker AH, Lange BJ, et al. Association of CYP3A4 genotype with treatment-related leukemia. Proc Natl Acad Sci U S A 1998; 95: 13176–81
Silverman LB, Gelber RD, Kimball Dalton V, et al. Improved outcomes for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 2001; 97: 1211–8
Laverdiere C, Chiasson S, Costea I, et al. Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood 2002; 100: 3832–4
Labuda D, Krajinovic M, Richer C, et al. Rapid detection of CYP1A1, CYP2D6, and NAT variants by multiplex polymerase chain reaction and allele-specific oligonucleotide assay. Anal Biochem 1999; 275: 84–92
Bourgeois S, Labuda D. Dynamic allele-specific oligonucleotide hybridization on solid support (DASO). Anal Biochem 2004; 324: 309–11
Krajinovic M, Labuda D, Sinnett D. Glutathione S-transferase P1 genetic polymorphisms and susceptibility to childhood acute lymphoblastic leukaemia. Pharmacogenetics 2002; 12: 655–8
Stevens A, Ray DW, Zeggini E, et al. Glucocorticoid sensitivity is determined by a specific glucocorticoid receptor haplotype. J Clin Endocrinol Metab 2004; 89: 892–7
Hongo T, Yamada S, Yajima S, et al. Biological characteristics and prognostic value of in vitro three-drug resistance to prednisolone, L-asparaginase, and vincristine in childhood acute lymphoblastic leukemia. Int J Hematol 1999; 70: 268–77
Kaspers GJ, Veerman AJ, Pieters R, et al. In vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Blood 1997; 90: 2723–9
Bailey S, Hall AG, Pearson AD, et al. Glucocorticoid resistance and the AP-1 transcription factor in leukaemia. Adv Exp Med Biol 1999; 457: 615–9
Huizenga NA, Koper JW, de Lange P, et al. Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab 1998; 83: 47–54
Lamberts SW, Huizenga AT, de Lange P, et al. Clinical aspects of glucocorticoid sensitivity. Steroids 1996; 61: 157–60
Pascussi JM, Gerbal-Chaloin S, Drocourt L, et al. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta 2003; 1619: 243–53
El-Sankary W, Plant NJ, Gibson GG, et al. Regulation of the CYP3A4 gene by hydrocortisone and xenobiotics: role of the glucocorticoid and pregnane X receptors. Drug Metab Dispos 2000; 28: 493–6
Aplenc R, Glatfelter W, Han P, et al. CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. Br J Haematol 2003; 122: 240–4
Lin RC, Wang WY, Morris BJ. High penetrance, overweight, and glucocorticoid receptor variant: case-control study. BMJ 1999; 319: 1337–8
Lin RC, Wang XL, Morris BJ. Association of coronary artery disease with glucocorticoid receptor N363S variant. Hypertension 2003; 41: 404–7
Dobson MG, Redfern CP, Unwin N, et al. The N363S polymorphism of the glucocorticoid receptor: potential contribution to central obesity in men and lack of association with other risk factors for coronary heart disease and diabetes mellitus. J Clin Endocrinol Metab 2001; 86: 2270–4
van Rossum EF, Koper JW, van den Beld AW, et al. Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf) 2003; 59: 585–92
Di Blasio AM, van Rossum EF, Maestrini S, et al. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol (Oxf) 2003; 59: 68–74
Griffin TC, Shuster JJ, Buchanan GR, et al. Slow disappearance of peripheral blood blasts is an adverse prognostic factor in childhood T cell acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Leukemia 2000; 14: 792–5
Schwartz CL, Thompson EB, Gelber RD, et al. Improved response with higher corticosteroid dose in children with acute lymphoblastic leukemia. J Clin Oncol 2001; 19: 1040–6
Acknowledgements
We are indebted to all the patients and their parents who consented to participate in this study. We are grateful to our colleagues Damian Labuda for discussion and biological material, Mark Bernstein for facilitating access to clinical data, and Alan Lovell for critical reading of the manuscript.
Isabelle Fleury has a studentship, and Maja Krajinovic and Daniel Sinnett scholarships from the Fonds de la recherche en santé du Québec. The Cancer Research Society, Inc. and Centre de Recherche, Hôpital Ste-Justine supported this study.
The authors have no conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fleury, I., Primeau, M., Doreau, A. et al. Polymorphisms in Genes Involved in the Corticosteroid Response and the Outcome of Childhood Acute Lymphoblastic Leukemia. Am J Pharmacogenomics 4, 331–341 (2004). https://doi.org/10.2165/00129785-200404050-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129785-200404050-00006