Abstract
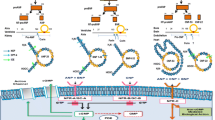
Cardiac glycosides have been used for decades to treat congestive heart failure. The recent identification of cardiotonic steroids such as ouabain, digoxin, marinobufagenin, and telocinobufagin in blood plasma, adrenal glands, and hypothalamus of mammals led to exciting new perspectives in the pathology of heart failure and arterial hypertension. Biosynthesis of ouabain and digoxin occurs in adrenal glands and is under the control of angiotensin II, endothelin, and epinephrine released from cells of the midbrain upon stimulation of brain areas sensing cerebrospinal Na+ concentration and, apparently, the body’;s K+ content. Rapid changes of endogenous ouabain upon physical exercise may favor the economy of the heart by a rise of intracellular Ca2+ levels in cardiac and atrial muscle cells. According to the sodium pump lag hypothesis, this may be accomplished by partial inhibition of the sodium pump and Ca2+ influx via the Na+/Ca2+ exchanger working in reverse mode or via activation of the Na+/K+-ATPase signalosome complex, generating intracellular calcium oscillations, reactive oxygen species, and gene activation via nuclear factor-κB or extracellular signal-regulated kinases 1 and 2. Elevated concentrations of endogenous ouabain and marinobufagenin in the subnanomolar concentration range were found to stimulate proliferation and differentiation of cardiac and smooth muscle cells. They may have a primary role in the development of cardiac dysfunction and failure because (i) offspring of hypertensive patients evidently inherit elevated plasma concentrations of endogenous ouabain; (ii) such elevated concentrations correlate positively with cardiac dysfunction, hypertrophy, and arterial hypertension; (iii) about 40% of Europeans with uncomplicated essential hypertension show increased concentrations of endogenous ouabain associated with reduced heart rate and cardiac hypertrophy; (iv) in patients with advanced arterial hypertension, circulating levels of endogenous ouabain correlate with BP and total peripheral resistance; (v) among patients with idiopathic dilated cardiomyopathy, high circulating levels of endogenous ouabain and marinobufagenin identify those individuals who are predisposed to progressing more rapidly to heart failure, suggesting that endogenous ouabain (and marinobufagenin) may contribute to toxicity upon digoxin therapy.
In contrast to endogenous ouabain, endogenous marinobufagenin may act as a natriuretic substance as well. It shows a higher affinity for the ouabain-insensitive α1 isoform of Na+/K+-ATPase of rat kidney tubular cells and its levels are increased in volume expansion and pre-eclampsia. Digoxin, which is synthesized in adrenal glands, seems to counteract the hypertensinogenic action of ouabain in rats, as do antibodies against ouabain, for example, (Digibind®) and rostafuroxin (PST 2238), a selective ouabain antagonist. It lowers BP in ouabain- and adducin-dependent hypertension in rats and is a promising new class of antihypertensive medication in humans.





Similar content being viewed by others
Notes
The use of trade marks is for purposed of product identification only and does not imply product endorsement.
References
Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature 2002; 415: 206–12.
Bers DM. Cardiac excitation-contraction coupling. Nature 2002; 415: 198–205.
Marbán E. Cardiac channelopathies. Nature 2002; 415: 213–8.
Towbin JA, Bowles NE. The failing heart. Nature 2002; 415: 227–33.
Dulin BR, Krum H. Drug therapy of chronic heart failure in the elderly: the current state of clinical-trial evidence. Curr Opin Cardiol 2006; 21: 393–9.
Aronow WS. Epidemiology, pathophysiology, prognosis, and treatment of systolic and diastolic heart failure. Cardiol Rev 2006; 14: 108–24.
Braunwald E. Effects of digitalis on the normal and the failing heart. J Am Coll Cardiol 1985; 5: 51A–9A.
Blaustein MP, Zhang J, Chen L, et al. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol 2006; 290: 514–23.
Gheorghiade M, Ferguson D. Digoxin: a neurohormone modulator in heart failure? Circulation 1991; 84: 2181–6.
Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006; 27: 178–86.
The Digitalis Investigation Group. The effect of digoxin on mortality and mobidity in patients with heart failure. New Engl J Med 1997; 336: 525–33.
Brophy JM. Rehabilitating digoxin. Eur Heart J 2006; 27: 127–9.
Haddy FJ. Role of dietary salt in hypertension. Life Sci 2006; 79: 1585–92.
Hamlyn JM, Ringel R, Schaeffer J, et al. A circulating inhibitor of Na+-K+-ATPase associated with essential hypertension. Nature 1982; 300: 650–2.
Moreth K, Kuske R, Renner D, et al. Blood pressure in essential hypertension correlates with the concentration of a circulating inhibitor of the sodium pump. Klin Wochenschr 1986; 64: 239–44.
Reichstein T. Cardenolid- und Pregnanglykoside. Die Naturwissenschaften 1967; 3: 53–76.
Höriger N, Zivanov D, Linde HH, et al. Cardenolide hydrogen suberates and other bufadienolide hydrogen suberates in Ch’an Su. Helv Chim Acta 1970; 53: 1993–2002.
Hamlyn JM, Blaustein MP, Bova S, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A 1991; 88: 6259–63.
Mathews WR, DuCharme DW, Hamlyn JM, et al. Mass spectral characterization of an endogenous digitalis like factor from human plasma. Hypertension 1991; 17: 930–5.
Schneider R, Wray V, Nimtz M, et al. Bovine adrenals contain, in addition to ouabain, a second inhibitor of the sodium pump. J Biol Chem 1998; 273: 784–92.
Kawamura A, Guo J, Itagaki Y, et al. On the structure of endogenous ouabain. Proc Natl Acad Sci U S A 1999; 96: 6654–9.
Komiyama Y, Nishimura N, Munakata M, et al. Identification of endogenous ouabain in culture supernatant of PC12 cells. J Hypertens 2001; 19: 229–36.
Boulanger BR, Lilly MP, Hamlyn JM, et al. Ouabain is secreted by the adrenal gland of the awake dogs. Am J Physiol 1993; 264: E413–9.
Masugi F, Ogihara T, Hasegawa T, et al. Normalization of high plasma level of ouabain-like immunoreactivity in primary aldosteronism after removal of adenoma. J Hum Hypertens 1988; 2: 17–20.
Laredo J, Hamilton JP, Hamlyn JM. Secretion of endogenous ouabain from bovine adrenal cells: role of zona glomerulosa and zona fasciculata. Biochem Biophys Res Commun 1995; 212: 487–93.
Manunta P, Evans G, Hamilton BP, et al. A new syndrome with elevated plasma ouabain and hypertension secondary to an adrenocortical tumor [abstract]. J Hypertens 1992; 10 Suppl. 4: S27.
Komiyama Y, Nishimura N, Munakata M, et al. Increases in plasma ouabainlike immunoreactivity during surgical extirpation of pheochromocytoma. Hypertens Res 1999; 22: 135–9.
Qazzaz HM, El-Masri MA, Valdes RJ. Secretion of a lactone-hydrogenated ouabain-like effector of sodium, potassium-adenosine triphosphatase activity by adrenal cells. Endocrinology 2000; 141: 3200–9.
Perrin A, Brasmes B, Chambaz EM, et al. Bovine adrenocortical cells in culture synthesize an ouabain-like compound. Molec Cell Endocrinol 1997; 126: 7–15.
Doris PA, Hayward-Lester A, Bourne D, et al. Ouabain production by cultured adrenal cells. Endocrinology 1996; 137: 533–9.
Laredo J, Hamilton BP, Hamlyn JM. Ouabain is secreted by bovine adrenocortical cells. Endocrinology 1994; 135: 794–7.
Hamlyn JM, Lu Z, Manunta P, et al. Observations on the nature, biosynthesis, secretion and significance of endogenous ouabain. Clin Exptl Hypertens 1998; 20: 523–33.
Lichtstein D, Steinitz M, Gati I, et al. Biosynthesis of digitalis-compound in rat adrenal cells: hydroxycholesterol as a precursor. Life Sci 1998; 62: 2109–26.
Malawista I, Davidson AE. Isolation and identification from rabbit skin. Nature 1961; 192: 871–2.
Laredo J, Shah JR, Lu Z, et al. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenal cortical cells via angiotensin II receptors. Hypertension 1997; 29: 401–407.
Laredo J, Shah JR, Hamilton BP, et al. Alpha-1 adrenergic receptors stimulate secretion of endogenous ouabain from human and bovine adrenocortical cells. In: Taniguchi K, Kayas S, editors. Na/K-ATPase and related ATPases. Amsterdam: Elsevier Science, 2000: 671–679.
Hiyama T, Watanabe E, Okado H, et al. The subfornical organ is the primary locus of sodium-level sensing by Nax sodium channels for the control of salt-intake behavior. J Neurosci 2004; 24: 2004; 24: 9276–81.
Wang H, White R, Leenen FHH. Stimulation of brain Na+ channels by FMRFamide in Dahl SS and SR rats. Am J Physiol Heart Circ Physiol 2003; 285: H2013–8.
Huang BS, Amin MS, Leenen FHH. The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol 2006; 21: 295–304.
Fedorova OV, Agalakova NI, Talan MI, et al. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J Hypertens 2005; 23: 1515–23.
Xie Z, Askari A. Na+/K+-ATPase as a signal inducer. Eur J Biochem 2002; 269: 2434–9.
Xie Z, Cai T. Na+-K+-ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv 2003; 3: 157–68.
Akimova O, Tremblay J, Hamet P, et al. The Na+/K+-ATPase as [K+]o sensor: role in cardiovascular disease pathogenesis and augmented production of endogenous cardiotonic steroids. Pathophysiology 2006; 13: 209–16.
Manunta P, Hamilton J, Rogowski AC, et al. Chronic hypertension induced by ouabain but not digoxin in the rat: antihypertensive effect of digoxin and digitoxin. Hypertens Res 2000; 23: S77–85.
Huang BS, Kudlac M, Kumarathasan R, et al. Digoxin prevents ouabain and high salt intake-induced hypertension in rats with sinoaortic denervation. Hypertension 1999; 34: 733–8.
Huang BS, Harmsen E, Yu H, et al. Brain ouabain-like activity and the sympathoexcitatory and pressor effects of central sodium in rats. Circ Res 1992; 71: 1059–66.
Sopucleous A, Elmatzoglou I, Souvatzoglou A. Ciculating endogenous digitalis-like factor(s) (EDLF) in man is derived from the adrenals and its secretion is ACTH-dependent. J Endocrinol Invest 2003; 26: 668–74.
Yamada K, Goto A, Omata M. Adrenocorticotropin-induced hypertension in rats: role of ouabain-like compound. Am J Hypertens 1997; 10: 403–8.
Huang BS, Cheung WJ, Wang H, et al. Activation of the brain renin-angiotensinaldosterone system by central sodium in Wistar rats. Am J Physiol Heart Circ Physiol 2006; 291: H1 109–17.
Di Filippo C, Filippelli A, Rinaldi B, et al. Chronic peripheral ouabain treatment affects the brain endothelin system of rats. J Hypertens 2003; 21: 747–53.
D’Amico M, Di Filippo C, Piegari E, et al. ETA endothelin receptors are involved in the ouabain-induced haemodynamic effects in the periaqueductal gray area of rats. Life Sci 2003; 72: 2211–8.
Goto A, Ishiguro T, Yamada K, et al. Isolation of an urinary digitalis-like factor indistinguishable from digoxin. Biochem Biophys Res Commun 1990; 173: 1093–101.
Goto A, Yamada K. Purifcation of endogenous digitalis-like factors from normal human urine. Clin Exp Hypertens 1998; 20: 551–6.
Qazzaz HMAM, Goudy SL, Valdes RJ. Deglycosylated products of endogenous digoxin-like immunoreactive factor in mammalian tissue. J Biol Chem. 1996; 271: 8731–7.
Goto A, Yamada K. Ouabain-like factor. Curr Opin Nephrol Hypertens 1998; 7: 189–96.
Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem 2002; 269: 2440–8.
Qazzaz HM, Cao Z, Bolanowski DD, et al. De novo biosynthesis and radiolabeling of mammalian digitalis-like factors. Clin Chem 2004; 50: 612–20.
Qazzaz HMA, Lane AN, Valdes RJ. Structural identification of digoxin-like factors (DLIFs) using NMR spectroscopy [abstract]. Clin Chem 2003; 49 Suppl. 6: A130.
Bagrov AY, Fedorova OV, Dmitrieva RI, et al. Characterization of a urinary bufodienolide Na+, K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension 1998; 31: 1097–103.
Komiyama Y, Dong XH, Nishimura N, et al. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem 2005; 38: 36–45.
Dmitrieva RI, Bagrov AY, Lalli E, et al. Mammalian bufadienolide is synthesized from cholesterol in the adrenal cortex by a pathway that is independent of cholesterol side-chain cleavage. Hypertension 2000; 36: 442–8.
Lichtstein D, Gati I, Samuelov S, et al. Identification of digitalis-like compounds in human cataractous lenses. Eur J Biochem 1993; 216: 261–8.
Sich B, Kirch U, Tepel M, et al. Pulse pressure correlates with a proscillaridin A immunoreactive compound. Hypertension 1996; 27: 1073–8.
Oda M, Kurosawa M, Numazawa S, et al. Determination of bufalin-like immunoreactivity in serum of humans and rats by time-resolved fluoroimmunoassay for using a monoclonal antibody. Life Sci 2001; 68: 1107–17.
Bova S, Blaustein MP, Ludens J, et al. Effect of an endogenous ouabainlike compound on heart and aorta. Hypertension 1991; 17: 944–50.
Schoner W, Bauer N, Müller-Ehmsen J, et al. Ouabain as a mammalian hormone. Ann N Y Acad Sci 2003; 986: 678–84.
Goto A, Yamada K, Nagoshi H, et al. Stress-induced elevation of ouabainlike compound in rat plasma and adrenal. Hypertension 1995; 26: 1173–6.
Manunta P, Stella P, Rivera R, et al. Left ventricular mass, stroke volume and ouabain-like factor in essential hypertension. Hypertension 1999; 34: 450–6.
Wang JG, Staessen JA, Messaggio E, et al. Salt, endogenous ouabain and blood pressure interactions in the general population. J Hypertens 2003; 21: 1475–81.
Pierdomenico SD, Bucci A, Manunta P, et al. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am J Hypertens 2001; 14: 44–50.
DiBartolo V, Balzan S, Pieraccini L, et al. Evidences for an ouabain-like immunoreactive factor in human newborn plasma coeluting with ouabain on HPLC. Life Sci 1995; 57: 1417–25.
Pidgeon GB, Richards AM, Nicholls MG, et al. Acute effects of intravenous ouabain in healthy volunteers. Clin Sci (Lond) 1994; 86: 391–7.
Rossoni LV, dos Santos L, Barker LA, et al. Ouabain changes arterial blood pressure and vascular reactivity to phenylephrine in L-NAME-induced hypertension. J Cardiovasc Pharmacol 2003; 41: 105–16.
Yuan CM, Manunta P, Hamlyn JM, et al. Long-term ouabain administration produces hypertension in rats. Hypertension 1993; 22: 178–87.
Pamnani MB, Chen S, Yuan CM, et al. Chronic blood pressure effects of bufalin, a sodium-potassium ATPase inhibitor in rats. Hypertension 1994; 23 Suppl. 1: I106–I109.
Aydemir-Koksoy A, Abramowitz J, Allen J. Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem 2001; 276: 46605–11.
Abramowitz J, Dai C, Hirschi KK, et al. Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and rat vascular smooth muscle cell line, A7r5. Circulation 2003; 108: 3048–53.
Gottlieb SS, Rogowski AC, Weinberg M, et al. Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation 1992; 86: 420–5.
Balzan S, Neglia D, Ghione S, et al. Increased circulating levels of ouabain-like factor in patients with asymptomatic left ventricular dysfunction. Eur J Heart Fail 2001; 3: 165–71.
Bagrov AY, Fedorova OV, Maslova MN, et al. Endogenous plasma Na, K-ATPase inhibitory activity and digoxin-like immunoreactivity after acute myocardial infarction. Cardiovasc Res 1991; 25: 371–7.
Manunta P, Ferrandi M. Different effects of marinobufagenin and endogenous ouabain. J Hypertens 2004; 257: 257–9.
Pitzalis MV, Hamlyn JM, Messaggio E, et al. Independent and incremental prognostic value of endogenous ouabain in idiopathic dilated cardiomyopathy. Eur J Heart Fail 2006; 8: 179–86.
Manunta P, Ferrandi M. Cardiac glycosides and cardiomyopathy. Hypertension 2006; 47: 343–4.
Pamnani M, Burris J, JF J, et al. Humoral Na-K pump inhibitory activity in essential hypertension and in normotensive subjects after acute volume expansion. Am J Hypertension 1989; 2: 524–31.
MacGregor G, Fenton S, Alaghband-Zadeh J, et al. An increase in circulating inhibitor of Na, K-dependent ATPase: a possible link between salt intake and the development of hypertension. Clin Sci 1981; 61: 17s–20s.
Poston L, Sewell R, Wilkinson S, et al. Evidence for a circulating sodium transport inhibitor in essential hypertension. Brit Med J 1981; 282: 847–9.
Blaustein M. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol 1993; 264: C1367–87.
Yamada K, Goto A, Nagoshi H, et al. Elevation of ouabainlike compound levels with hypertonic sodium chloride load in rat plasma and tissues. Hypertension 1997; 30: 94–8.
Manunta P, Hamilton BP, Hamlyn JM. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. Am J Physiol Regul Integr Comp Physiol 2006; 290: R553–9.
Manunta P, Messaggio E, Ballabeni C, et al. Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension 2001; 38: 198–203.
Scheiner-Bobis G, Schoner W. A fresh facet for ouabain action. Nat Med 2001; 7: 1288–9.
Manunta P, Rogowski AC, Hamilton BP, et al. Ouabain-induced hypertension in the rat: Relationships among plasma and tissue ouabain and blood pressure. J Hypertens 1994; 12: 549–60.
Veerasingham SJ, Leenen FH. Ouabain- and central sodium-induced hypertension depend on the ventral anteroventral third ventricle region. Am J Physiol 1999; 276: H63–70.
Rossoni LV, Salaices M, Marin J, et al. Alterations in phenylephrine-induced contractions and the vascular expression of Na+, K+-ATPase in ouabain-induced hypertension. Br J Pharmacol 2002; 135: 771–81.
Rossoni L, Salaices M, Miguel M, et al. Ouabain-induced hypertension is accompanied by increases in endothelial vasodilator factors. Am J Physiol Heart Circ Physiol 2002; 283: H2110–8.
Cheung WJ, Kent MA, El-Shahat E, et al. Central and peripheral renin-angiotensin systems in ouabain-induced hypertension. Am J Physiol Heart Cire Physiol 2006; 291: H624–30.
Padilha AS, Rossoni LV, Xavier FE, et al. Ouabain at nanomolar concentration promotes synthesis and release of angiotensin II from the endothelium of the tail vascular bed of spontaneously hypertensive rats. J Cardiovasc Pharmacol 2004; 44: 372–80.
Ferrandi M, Molinari I, Barassi P, et al. Organ hypertrophie signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem 2004; 279: 33306–14.
Kimura K, Manunta P, Hamilton BP, et al. Different effects of in vivo ouabain and digoxin on renal artery function and blood pressure in rats. Hypertens Res 2000; 23 Suppl.: S67–76.
Manunta P, Hamilton BP, Hamlyn JM. Structure-activity relationship for hypertensinogenic activity of ouabain. Role of the sugar and lactone ring. Hypertension 2001; 37: 472–7.
Rossi GP, Manunta P, Hamlyn JM, et al. Immunoreactive endogenous ouabain in primary hyperaldosteronism and essential hypertension: relationship with plasma renin, aldosterone and blood pressure levels. J Hypertens 1995; 13: 1181–91.
Manunta P, Iacoviello M, Forleo C, et al. High circulating levels of endogenous ouabain in the offspring of hypertensive and normotensive individuals. J Hypertens 2005; 23: 1677–81.
Ferrandi M, Barassi P, Molinari I, et al. Ouabain antagonists as antihypertensive agents. Curr Pharm Des 2005; 11: 3301–5.
Lanzani C, Citterio L, Jankaricova M, et al. Role of the adducin family genes in human essential hypertension. J Hypertens 2005; 23: 543–9.
Ferrari P, Ferrandi M, Valentini G, et al. Rostafuroxin: an ouabain antagonist that corrects renal and vascular Na+-K+-ATPase alterations in ouabain and adducin-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 2006; 290: R529–35.
Huang L, Li H, Xie Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J Mol Cell Cardiol 1997; 29: 429–4371.
Aizman O, Uhlén P, Lal M, et al. Ouabain, a steroid hormone that signals with slow calcium oscillations. PNAS 2001; 98: 13420–4.
Müller-Ehmsen J, Juvvadi P, Thompson CB, et al. Ouabain and substrate affinities of human Na+-K+-ATPase α1β1, α2βl, and α3β1 when expressed separately in yeast cells. Am J Physiol Cell Physiol 2001; 281: C1355–64.
Crambert G, Haseler U, Beggah A, et al. Transport and pharmacological properties of nine different human Na, K-ATPase isoenzymes. J Biol Chem 2000; 275: 1976–86.
Wang J, Velotta JB, McDonough AA, et al. All human Na+-K+-ATPase a-subunit isoforms have a similar affinity for cardiac glycosides. Am J Physiol 2001; 281: C1336–43.
James P, Grupp I, Grupp G, et al. Identification of a specific role for the Na, K-ATPase α2 isoforms a regulator of calcium in the heart. Mol Cell 1999; 3: 555–63.
He S, Shelly D, Moseley A, et al. The α1 and α2-isoformes of Na+, K+-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol 2001; 281: R917–25.
Dostanic-Larson I, Lorenz JN, Van Huysse JW, et al. Physiological role of the α1-and α2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol 2006; 290: R524–8.
Dostanic I, Paul RJ, Lorenz JN, et al. The α2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 2005; 288: H477–85.
Haas M, Wang H, Tian J, et al. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem 2002; 277: 18694–18702.
Jäger H, Wozniak G, Akintürk I-H, et al. Expression of sodium pump isoforms and other sodium or calcium ion transporters in the heart of hypertensive patients. Biochim Biophys Acta 2001; 1513: 149–59.
Schwinger RH, Bubdgaard H, Müller-Ehmsen J, et al. The Na, K-ATPase in the failing human heart. Cardiovasc Res 2003; 57: 913–20.
Zahler R, Gilmore-Hebert M, Baldwin J, et al. Expression of alpha isoforms of the Na, K-ATPase in human heart. Biochim Biophys Acta 1993; 1149: 189–94.
Shamraj O, Grupp I, Grupp G, et al. Characterization of Na/K-ATPase, its isoforms, and the inotropic response to ouabain in isolated failing hearts. Cardiovasc Res 1993; 72: 2229–37.
Schwinger RHG, Wang J, Frank K, et al. Reduced sodium pump α1, α3, and β1-isoform protein levels and Na+, K+-ATPase activity but unchanged Na+-Ca2+ exchanger protein levels in human heart failure. Circulation 1999; 99: 2105–12.
Schwinger R, Muller-Ehmsen J, Frank K, et al. Enhanced sensitivity of the failing human myocardium to cardiac glycosides and Na+-channel activators. Am Heart J 1996; 131: 988–93.
Wang H, Yuan W-Q, Lu ZR. Differential regulation of the sodium pump alpha-subunit isoform gene by ouabain and digoxin in tissues of rats. Hypertens Res 2000; 23: S55–60.
Müller-Ehmsen J, Nickel J, Zobel C, et al. Longer term effects of ouabain on the contractility of rat isolated cardiomyocytes and on the expression of Ca and Na regulating proteins. Basic Res Cardiol 2003; 98: 90–6.
Vermuri R, Longoni S, Philipson K. Ouabain treatment of cardiac cells induced enhanced Na+-Ca2+ exchange activity. Am J Physiol 1989; 256: C1273–6.
Yamada K, Goto A, Hui C, et al. Role of ouabain-like compound in the regulation of plasma aldosterone concentration in rats. Life Sci 1996; 38: 1633–837.
Adair CD, Buckalew V, Taylor K, et al. Elevated endoxin-like factor complicating a multifetal second trimester pregnancy: treatment with digoxin-binding immunoglobulin. Am J Nephrol 1996; 16: 529–31.
Goodlin RC. Antidigoxin antibodies in eclampsia. New Engl J Med 1988; 318: 518–9.
Ferrari P, Torielli L, Ferrandi M, et al. PST 2238: a new antihypertensive compound that antagonizes the long term pressor effect of ouabain. J Pharmacol Exptl Therapeut 1998; 285: 83–94.
Huang BS, Van Vliet BN, Leenen FHH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on high salt diet. Am J Physiol Heart Circ Physiol 2004; 287: Hl 160–6.
Leenen FHH, Harmsen E, Yu H. Dietary sodium and central vs peripheral ouabain-like activity in Dahl salt-sensitive vs salt-resistant rats. Am J Physiol 1994; 267: H1916–20.
Huang BS, Wang H, Leenen FHH. Enhanced sympathoexcitatory and pressor responses to central Na+ in Dahl salt-sensitive vs -resistant rats. Am J Physiol Heart Circ Physiol 2001; 281: H1881–9.
Leenen FHH, Harmsen E, Yu H, et al. Dietary sodium stimulates ouabainlike activity in adrenalectomized spontaneously hypertensive rats. Am J Physiol 1993; 265: H421–4.
Huang BS, Leenen FHH. Both brain angiotensin II and ‘ouabain’ contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 1998; 32: 1028–33.
Takahashi H, Matsuzawa M, Okabayashi H, et al. Evidence for digitalis-like substance in the hypothalamopituitary axis in rats: implications in the central cardiovascular regulation associated with excess intake of sodium. Jpn Circ J 1987; 51: 1199–207.
Huang BS, Ganten D, Leenen FHH. Responses to central Na+ and ouabain are attenuated in transgenic rats deficient in brain angiotensinogen. Hypertension 2001; 37: 683–6.
Huang BS, Leenen FHH. Sympathoexcitatory and pressor responses to increased brain sodium and ouabain are mediated via brain angiotensin II. Am J Heart Circ Physiol 1996; 270: H275–80.
Huang BS, Leenen FHH. Brain ‘ouabain’ mediates the sympathoexcitatory and hypertensive effects of high sodium intake in Dahl salt-sensitive rats. Circ Res 1994; 74: 586–95.
Huang BS, Leenen FHH. Brain ‘ouabain’ and angiotensin ii in salt-sensitive hypertension in spontaneously hypertensive rats. Hypertension 1996; 28: 1005–12.
Budzikowski AS, Leenen FHH. Brain ‘Ouabain’ in the median preoptic nucleus mediates sodium-sensitive hypertension in spontaneously hypertensive rats. Hypertension 1997; 29: 599–605.
Aileru AA, De Albuquerque A, Hamlyn JM, et al. Synaptic plasticity in sympathetic ganglia from acquired and inherited forms of ouabain-dependent hypertension. Am J Physiol Regul Integra Comp Physiol 2001; 281: R635–44.
Hamlyn JM, Manunta P. Ouabain, digitalis like factors and hypertension. J Hypertens Suppl 1992; 10: S99–S111.
Huang BS, Huang X, Harmsen E, et al. Chronic central versus peripheral ouabain, blood pressure, and sympathetic activity in rats. Hypertension 1994; 23: 1087–90.
Wang H, Huang BS, Leenen FHH. Brain sodium channel and ouabainlike compounds mediate central aldosterone-induced hypertension. Am J Heart Circ Physiol 2003; 285: H2516–23.
Wang H, Leenen FHH. Brain sodium channels mediate increases in brain ‘ouabain’ and blood pressure in Dahl S rats. Hypertension 2002; 40: 96–100.
Zhao X, White R, Huang BS, et al. High salt intake and the brain renin-angiotensin system in Dahl salt-sensitive rats. J Hypertens 2001; 19: 89–98.
Berendes E, Cullen P, Van Aken H, et al. Endogenous glycosides in critically ill patients. Crit Care Med 2003; 31: 1331–7.
Paganelli F, Maixent J-M, Gélisse R, et al. Effect of digoxin on chemoreflex in patients with chronic heart failure. Cell Molec Biol 2001; 47 (2): 335–40.
Valdes Jr R. Endogenous digoxin-immunoactive factor in human subjects. Fed Proc 1985; 44: 2800–5.
Wang H, Huang BS, Ganten D, et al. Prevention of sympathetic and cardiac dysfunction after myocardial infarction in transgenic rats deficient in brain angiotensinogen. Circ Res 2004; 94: 843–9.
Fedorova OV, Bagrov AY. Inhibition of Na/K-ATPase from rat aorta by two endogenous Na/K pump inhibitors, ouabain and marinobufagenin: evidence of interaction with different a subunit isoforms. Am J Hypertens 1997; 10: 929–35.
Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na, K pump ligands are differentially regulated during acute NaCl loading of DAHL rats. Circulation 2000; 102: 3009–14.
Fedorova OV, Kolodkin NI, Agalakova NI, et al. Marinobufagenin, an endogenous α-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension 2001; 37: 462–6.
Blanco G, Mercer RW. Isozymes of the Na-K ATPase: heterogeneity in structure, diversity in function. Am J Physiol 1998; 275: F633–50.
Bagrov AY, Feodorova OV, Dmitrieva RI, et al. Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anaesthetized dogs. Cardiovasc Res 1996; 31: 296–305.
Lopatin DA, Ailamazian EK, Dmitrieva RI, et al. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens 1999; 17: 1179–87.
Hop VV, Ianosi-Irimie MR, Pridjian CA, et al. Involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol 2005; 25: 520–8.
Vu H, Ianosi-Irimie M, Danchuk S, et al. Resibufogenin corrects hypertension in a rat model of human preeclampsia. Exp Biol Med 2006; 231: 215–20.
Xie J-T, Chen W, January CT. Resibufogenin obtained from toad venoms and its pharmacological and toxicological effects. Meth Find Clin Pharmacol 1993; 15: 689–97.
Fridman AI, Matveev SA, Agalakova NA, et al. Marinobufagenin, an endogenous ligand of alpha-1 sodium pump, is a marker of congestive heart failure severity. J Hypertens 2002; 20: 1189–94.
Kennedy DJ, Vetteth S, Periyasamy SM, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 2006; 47: 488–95.
Fedorova OV, Talan MI, Agalakova NI, et al. Endogenous ligand of α1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride dependent hypertension. Circulation 2002; 105: 1122–7.
Fedorova OV, Kolodkin NI, Agalakova NI, et al. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J Hypertens 2005; 23: 835–42.
Bauer N, Müller-Ehmsen J, Krämer U, et al. Ouabain-like compound changes rapidly upon physical exercise in man and dog: effects of β-blockade and ACE-inhibition. Hypertension 2005; 45: 1024–8.
Erdmann E, Schoner W. Ouabain receptor interactions in (Na+ + K+)-ATPase preparations from different tissues and species: determination of kinetic constants and dissociation constants. Biochim Biophys Acta 1973; 307: 386–98.
Gao J, Wymore RS, Wang Y, et al. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J Gen Physiol 2002; 119: 297–312.
Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur J Biochem 2004; 271: 1054–62.
Balzan S, D’Urso G, Nicolini G, et al. Erythrocytes sodium pump stimulation by ouabain and an endogenous ouabain-like factor. Cell Biochem Funct 2007; 25: 297–303.
Khundmiri SJ, Metzler MA, Ameen M, et al. Ouabain induces cell proliferation through calcium dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 2006; 291: C1247–57.
Dmitrieva RI, Doris PA. Ouabain is a potent promoter of growth and activator of erk1/2 in ouabain-resistant rat renal epithelial cells. J Biol Chem 2003; 278: 28160–6.
Reuter H, Henderson SA, Han T, et al. The Na+-Ca2+ Exchanger is essential for the action of cardiac glycosides. Circ Res 2002; 90: 305–8.
Arnon A, Hamlyn JM, Blaustein MP. Ouabain augments Ca2+ transients in arterial smooth muscle without raising cytosolic Na+. Am J Physiol Heart Circ Physiol 2000; 279: H679–91.
Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity a subunit isoforms are differently distributed in cells. Proc Natl Acad Sci U S A 1997; 94: 1800–5.
Golovina VA, Song H, James PF, et al. Na+ pump α2-subunit expression modulates Ca2+ signaling. Am J Physiol Cell Physiol 2003; 284: C475–86.
Zhang J, Lee MY, Cavalli M, et al. Sodium pump α2 subunits control myogenic tone and blood pressure in mice. J Physiol 2005; 569: 243–56.
Song H, Lee MY, Kinsey SP, et al. An N-terminal sequence targets and tethers Na+ pump α2 subunits to specialized plasma membrane microdomains. J Biol Chem 2006; 281: 12929–40.
Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol 2000; 278: C163–73.
Lee MY, Song H, Nakai J, et al. Local subplasma membrane Ca2+ signals detected by a tethered Ca2+ sensor. PNAS 2006; 103: 13232–7.
Wasserstrom JA, Aistrup GL. Digitalis: new actions for an old drug. Am J Physiol Heart Circ Physiol 2005; 289: H1781–93.
Dostanic I, Lorenz JN, Schultz JEJ, et al. The α2 isoform of Na, K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem 2003; 278: 53026–34.
Iwamoto T, Kita S, Uehara A, et al. Molecular determinants of Na+/Ca2+ exchange (NCX1) inhibition by SEA0400. J Biol Chem 2004; 279: 7544–53.
Iwamoto T, Kita S, Zhang J, et al. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+ /Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 2004; 10: 1193–9.
Noble D. Mechanism of action of therapeutic levels of cardiac glycosides. Cardiovasc Res 1980; 14: 495–514.
Santana LF, Gómez AM, Lederer WJ. Ca2+ Flux through promiscuous cardiac Na+ channels: slip-mode conductance. Sci 1998; 279: 1027–33.
Dong XH, Komiyama Y, Nishimura N, et al. Nanomolar level of ouabain increases intracellular calcium to produce nitric oxide in rat aortic endothelial cells. Clin Exp Pharmacol Physiol 2004; 31: 276–83.
Zhu Z, Tepel M, Neusser M, et al. Low concentrations of ouabain increase cytosolic free calcium concentration in rat vascular smooth muscle cells. Clin Sci 1996; 90: 9–12.
Rosen H, Glukhman V, Feldmann T, et al. Cardiac steroids induce changes in recycling of the plasma membrane in human NT2 cells. Mol Biol Cell 2004; 15: 1044–54.
Liu L, Mohammadi K, Aynafshar B, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol 2003; 284: C1550–60,.
Liu J, Liang M, Liu L, et al. Ouabain-induced endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kid Int 2005; 67: 1844–54.
Zhang S, Malmersjö S, Li J, et al. Distinct role of the N-terminal tail of the Na, K-ATPase catalytic subunit as a signal transducer. J Biol Chem 2006; 281: 21954–62.
Miyakawa-Naito A, Uhlén P, Lal M, et al. Cell signaling microdomain with Na, K-ATPase and inositol 1,4,5-triphosphate receptor generates calcium oscillations. J Biol Chem 2003; 318: 50355–61.
Wang H, Haas M, Liang M, et al. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 2004; 279: 17250–9.
Yuan Z, Cai T, Tian J, et al. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell 2005; 16: 4034–45.
Oweis S, Wu L, Kiela PR, et al. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK1 cells. Am J Physiol Renal Physiol 2006; 290: F997–F1009.
Liang M, Cai T, Tian J, et al. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Bio Chem 2006; 281: 19709–19.
Daniel EE, El-Yazbi A, Cho WJ. Caveolae and calcium handling, a review and a hypothesis. J Cell Mol Med 2006; 10: 529–44.
Yudowski GA, Efendiev R, Pedemonte CH, et al. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. PNAS 2000; 97: 6556–61.
Ibarra FR, Jun Cheng SX, Agrén M, et al. Intracellular sodium modulates the state of protein kinase C phosphorylation of rat proximal tubule Na+, K+-ATPase. Acta Physiol Scand 2002; 175: 165–71.
Lecuona E, Ridge K, Pesce L, et al. The GTP-binding protein RhoA mediates Na, K ATPase exocytosis in alveolar epithelial cells. Molec Biol Cell 2003; 14: 3888–97.
Pesce L, Cornelias A, Sznajder JI. β-Adrenergic agonists regulate Na-K-ATPase via p70S6k. Am J Physiol Lung Cell Mol Physiol 2003; 285: L802–7.
Tian J, Gong X, Xie Z. Signal-transducing function of Na+/K+-ATPase is essential for ouabain’s effect on [Ca2+]i in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 2001; 281: H1899–907.
Mohammadi K, Kometiani P, Xie Z, et al. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem 2001; 276: 42050–6.
Mohammadi K, Liu L, Tian J, et al. Positive inotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. J Cardiovasc Pharmacol 2003; 41: 609–14.
Peng M, Huang L, Xie Z, et al. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expression of early response genes in cardiac myocytes. J Biol Chem 1996; 271: 10372–8.
Harwood SM, Allen DA, Raftery M, et al. Calpain is a mediator of myocardial injury in experimental uremia: is it activated by endogenous ouabain? Kidney Int 2003; 83 Suppl. 84: S177–80.
Lebart M-C, Benyamin Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J 2006; 273: 3415–26.
Liu J, Tian J, Haas M, et al. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem 2000; 275: 27838–44.
Huang L, Kometiani P, Xie Z. Differential regulation of Na/K-ATPase alpha-subunit isoform gene expressions in cardiac myocytes by ouabain and other hypertrophic stimuli. J Mol Cell Cardiol 1997; 29: 3157–67.
Kometiani P, Tian J, Li J, et al. Regulation of Na/K-ATPase α1-subunit gene expression by ouabain and other hypertrophic stimuli in neonatal rat cardiac myocytes. Mol Cell Biochem 2000; 215: 65–72.
Eva A, Kirch U, Scheiner-Bobis G. Signaling pathways involving the sodium pump stimulate NO production in endothelial cells. Biochim Biophys Acta 2006; 1758: 1809–14.
Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Mol Cell Biol 2006; 7: 589–600.
Trevisi L, Visentin B, Cusinato F, et al. Antiapoptotic effect of ouabain in human umbilical vein endothelial cells. Biochem Biophys Res Commun 2004; 321: 716–21.
Liu J, Kesirv R, Periyasamy S, et al. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PKI cells by a clathrin-dependent mechanism. Kidney Int 2004; 66: 227–41.
Chibalin AV, Ogimoto G, Pedemonte CH, et al. Dopamine-induced endocytosis of Na+, K+-ATPase is initiated by phosphorylation of Ser-18 in the rat a subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem 1999; 274: 1920–7.
Khundmiri SJ, Bertorello AM, Delamere NA, et al. Clathrin-mediated endocytosis of Na+, K+-ATPase in response to parathyroid hormone requires ERK-dependent phosphorylation of Ser-11 within the α1-subunit. J Biol Chem 2004; 279: 17418–27.
Efendiev R, Krmar RT, Ogimoto G, et al. Hypertension-linked mutation in the adducin α-subunit leads to higher AP2-μ2 phosphorylation and impaired Na+, K+-ATPase trafficking in response to GPCR signals and intracellular sodium. Circ Res 2004; 95: 1100–8.
Núnez-Duran H, Atonal F, Contreras P, et al. Endocytosis inhibition protects the isolated guinea pig heart against ouabain toxicity. Life Sci 1996; 58: 193–8.
Tian J, Cai T, Yuan Z, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell 2006; 17: 317–26.
Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal transducing function of Na+/K+-ATPase. J Biol Chem 2000; 275: 27832–7.
Kometiani P, Li J, Gnudi L, et al. Multiple signal transduction pathways link Na+/ K+-ATPase to growth-related genes in cardiac myocytes: the roles of Ras and mitogen-activated protein kinases. J Biol Chem 1998; 273: 15249–56.
Golomb E, Hill MR, Brown RG, et al. Ouabain enhances the mitogenic effect of serum in vascular smooth muscle cells. Am J Hypertens 1994; 7: 69–74.
Tian J, Liu J, Garlid KD, et al. Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes: a potential role for mitochondrial KATP channels. Mol Cell Biochem 2003; 242: 181–7.
Xie Z, Kometiani P, Liu J, et al. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem 1999; 274: 19323–8.
Li J, Zelenin S, Aperia A, et al. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-κB. J Am Soc Nephrol 2006; 17: 1848–57.
Golden W, Martin L. Low-dose ouabain protects against excitotoxic apoptosis and up-regulates nuclear Bcl-2 in vivo. Neuroscience 2006; 137: 133–44.
Al-Khalili L, Kotova O, Tsuchida H, et al. ERK1/2 mediates insulin stimulation of Na, K-ATPase by phosphorylation of the α-subunit in human skeletal muscle cells. J Biol Chem 2004; 279: 25211–8.
Michlig S, Mercier A, Doucet A, et al. ERK1/2 controls Na, K-ATPase activity and transepithelial sodium transport in the principal cell of the cortical collecting duct of the mouse kidney. J Biol Chem 2004; 279: 51002–12.
Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol 2005; 67: 929–36.
Li S, Wattenberg EV. Differential activation of mitogen-activated protein kinases by palytoxin and ouabain, two ligands for the Na+, K+-ATPase. Toxicol Appl Pharmacol 1998; 151: 377–84.
Kotova O, Al-Khalili L, Talia S, et al. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J Biol Chem 2006; 281: 20085–94.
Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev 2000; 10: 508–14.
Haq S, Choukroun G, Kang Z, et al. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol 2000; 151: 117–30.
Deutsch J, Jang HG, Mansur N, et al. 4-(3′αl5β,-dihydroxy-5′β,-estran-17′β,-y1)furan-2-methyl alcohol: an anti-digoxin agent with a novel mechanism of action. J Med Chem 2006; 49: 600–6.
Rocchetti M, Besana A, Mostacciuolo G, et al. Modulation of sarcoplasmic reticulum function by Na+/K+ pump inhibitors with different toxicity: digoxin and PST2744 [(E, Z)-3-((2-Aminoethoxy)imino)androstane-6,17-dione hydrochloride]. J Pharmacol Exptl Ther 2005; 313: 207–15.
Acknowledgments
Dr Schoner acknowledges grants from Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg, Fonds der Chemischen Industrie, Frankfurt/Main, and the Akademie für Tiergesundheit, Bonn, Germany. The authors have no conflict of interests relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schoner, W., Scheiner-Bobis, G. Endogenous and Exogenous Cardiac Glycosides and their Mechanisms of Action. Am J Cardiovasc Drugs 7, 173–189 (2007). https://doi.org/10.2165/00129784-200707030-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129784-200707030-00004