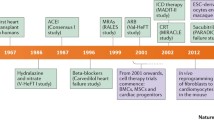
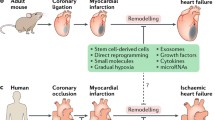
Abstract
Cardiomyocytes are terminally differentiated and are unable to proliferate in response to injury. Genetic modulation, cell transplantation and tissue engineering promise a revolutionary approach for myocardial regeneration and tissue repair after myocardial injury. Current data derived from animal models suggest that it may be possible to treat heart failure by inserting genetic materials or myogenic cells into injured myocardium. Success with animal models has raised the hope for new treatment after heart attacks and could prove an alternative to transplantation, particularly in elderly patients for whom there is often a lack of donor hearts. This exciting research, however, still faces significant difficulties before it can develop into a clinical therapeutic tool and many challenges need to be overcome before cell transplantation, gene therapy and tissue engineering can be considered efficient, therapeutic strategies for myocardial regeneration.



Similar content being viewed by others
References
Sharpe N, Doughty R. Epidemiology of heart failure and ventricular dysfunction. Lancet 1998; 352 Suppl. 1: SI3–7
Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J 1997; 133: 703–12
Hoes AW, Mosterd A, Grobbee DE. An epidemic of heart failure? Recent evidence from Europe. Eur Heart J 1998; 19 Suppl. L: L2–9
Cleland JG, Khand A, Clark A. The heart failure epidemic: exactly how big is it?. Eur Heart J 2001; 22: 623–6
Swedberg K, Kjekshus J, Snapinn S. Long-term survival in severe heart failure in patients treated with enalapril. Ten year follow-up of CONSENSUS I. Eur Heart J 1999; 20: 136–9
Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation 1999; 100: 999–1008
Reinlib L, Field L. Cell transplantation as future therapy for cardiovascular disease?: A workshop of the National Heart, Lung, and Blood Institute. Circulation 2000; 101: E182–7
Kessler PD, Byrne BJ. Myoblast cell grafting into heart muscle: cellular biology and potential applications. Annu Rev Physiol 1999; 61: 219–42
Soonpaa MH, Koh GY, Klug MG, et al. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium [see comments]. Science 1994; 264: 98–101
Koh GY, Kim SJ, Klug MG, et al. Targeted expression of transforming growth factor-beta 1 in intracardiac grafts promotes vascular endothelial cell DNA synthesis. J Clin Invest 1995; 95: 114–21
Leor J, Patterson M, Quinones MJ, et al. Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium?. Circulation 1996; 94: II332–6
Li RK, Jia ZQ, Weisel RD, et al. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg 1996; 62: 654–60; discussion 660–1
Li RK, Mickle DA, Weisel RD, et al. Natural history of fetal rat cardiomyocytes transplanted into adult rat myocardial scar tissue. Circulation 1997; 96: II-179-86; discussion 186-7
Li RK, Mickle DA, Weisel RD, et al. In vivo survival and function of transplanted rat cardiomyocytes. Circ Res 1996; 78: 283–8
Scorsin M, Hagege AA, Dolizy I, et al. Can cellular transplantation improve function in doxorubicin-induced heart failure?. Circulation 1998; 98: II151–5; discussion II155-6
Scorsin M, Hagege AA, Marotte F, et al. Does transplantation of cardiomyocytes improve function of infarcted myocardium?. Circulation 1997; 96: II188–93
Scorsin M, Marotte F, Sabri A, et al. Can grafted cardiomyocytes colonize peri-infarct myocardial areas?. Circulation 1996; 94: II337–40
Reinecke H, Zhang M, Bartosek T, et al. Survival, integration, and differentiation of cardiomyocyte grafts: A study in normal and injured rat hearts. Circulation 1999; 100: 193–202
Etzion S, Battler A, Barbash IM, et al. Influence of embryonic cardiomyocyte transplantation on the progression of heart failure in a rat model of extensive myocardial infarction. J Mol Cell Cardiol 2001; 33: 1321–30
Yoo KJ, Li RK, Weisel RD, et al. Heart cell transplantation improves heart function in dilated cardiomyopathic hamsters. Circulation 2000; 102: III204–9
Muller-Ehmsen J, Dow JS, Sakoda T, et al. Quantitative analysis of the survival of neonatal cardiomyocytes after grafting into healthy rat left ventricle using fluorescence based real-time TaqMan PCR [abstract]. Circulation 2000; 102 Suppl.: II–651
Zhang M, Methot D, Fujio Y, et al. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol 2001; 33: 907–21
Varda-Bloom N, Leor J, Ohad DG, et al. Cytotoxic T lymphocytes are activated following myocardial infarction and can recognize and kill healthy myocytes in vitro. J Mol Cell Cardiol 2000; 32: 2141–9
Hosenpud JD, Bennett LE, Keck BM, et al. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official report 1999. J Heart Lung Transplant 1999; 18: 611–26
Hooper TL, Stephenson LW. Cardiomyoplasty for end-stage heart failure. Surg Annu 1993; 25: 157–73
Chiu RC. Cardiac cell transplantation: the autologous skeletal myoblast implantation for myocardial regeneration. Adv Card Surg 1999; 11: 69–98
Murry CE, Wiseman RW, Schwartz SM, et al. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest 1996; 98: 2512–3
Pouzet B, Vilquin JT, Hagege AA, et al. Intramyocardial transplantation of autologous myoblasts: can tissue processing be optimized?. Circulation 2000; 102: III210–5
Dorfman J, Duong M, Zibaitis A, et al. Myocardial tissue engineering with autologous myoblast implantation. J Thorac Cardiovasc Surg 1998; 116: 744–51
Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation [published erratum appears in Nat Med 1998 Oct; 4 (10): 1200]. Nat Med 1998; 4: 929–33
Scorsin M, Hagege A, Vilquin JT, et al. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J Thorac Cardiovasc Surg 2000; 119: 1169–75
Menasche P, Hagege AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet 2001; 357: 279–80
Reinecke H, MacDonald GH, Hauschka SD, et al. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol 2000; 149: 731–40
Reinecke H, Murry CE. Transmural replacement of myocardium after skeletal myoblast grafting into the heart. Too much of a good thing?. Cardiovasc Pathol 2000; 9: 337–44
Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts [see comments] [published erratum appears in Science 1998 Dec 4; 282 (5395): 1827]. Science 1998; 282: 1145–7
Kahat I, Kenyagin-Karsenti D, Druckmann M, et al. Human embryonic stem cells can differentiate into myocytes portraying cardiomycytic structural and functional properties. J Clin Invest. In press
Shamblott MJ, Axelman J, Littlefield JW, et al. Human embryonic germ cell derivatives express a broad range of developmentally distinct markers and proliferate extensively in vitro. Proc Natl Acad Sci USA 2001; 98: 113–8
Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest 2000; 105: 1663–8
Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 1995; 18: 1417–26
Makino S, Fukuda K, Miyoshi S, et al. Cardiomyocytes can be generated from marrow stremai cells in vitro. J Clin Invest 1999; 103: 697–705
Shamblott MJ, Axelman J, Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA 1998; 95: 13726–31
Klug MG, Soonpaa MH, Koh GY, et al. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest 1996; 98: 216–4
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–7
Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 2000; 6: 1282–6
Cossu G, Mavilio F. Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective?. J Clin Invest 2000; 105: 1669–74
Wang JS, Shum-Tim D, Galipeau J, et al. Marrow stremai cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg 2000; 120: 999–1005
Tornita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 1999; 100: II247–56
Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001; 410: 701–5
Sussman M. Cardiovascular biologyHearts and bones. Nature 2001; 410: 640–1
Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–7
Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5: 434–8
Shintani S, Murohara T, Ikeda H, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 2001; 103: 897–903
Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 2001; 103: 634–7
Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7: 430–6
Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells. Nature 1997; 385: 810–3
Lanza RP, Cibelli JB, West MD. Prospects for the use of nuclear transfer in human transplantation. Nat Biotechnol 1999; 17: 1171–4
Humphreys D, Eggan K, Akutsu H, et al. Epigenetic instability in ES cells and cloned mice. Science 2001; 293: 95–7
Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999; 354 Suppl. 1: SI32–4
Leor J, Aboulafia-Etzion S, Dar A, et al. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium?. Circulation 2000; 102: III56–61
Akins RE, Boyce RA, Madonna ML, et al. Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells. Tissue Eng 1999; 5: 103–8
Bursac N, Papadaki M, Cohen RJ, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol 1999; 277: H433–44
Carrier RL, Papadaki M, Rupnick M, et al. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol Bioeng 1999; 64: 580–9
Papadaki M, Bursac N, Langer R, et al. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol 2001; 280: H168–78
Li RK, Jia ZQ, Weisel RD, et al. Survival and function of bioengineered cardiac grafts. Circulation 1999; 100: II63–9
Bonadio J, Smiley E, Patil P, et al. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration [see comments]. Nat Med 1999; 5: 753–9
Leor J, Prentice H, Salterelli V, et al. Gene transfer and cell transplant: an experimental approach to repair a ‘broken heart’. Cardiovasc Res 1997; 35: 431–1
Nabel EG. Gene therapy for cardiovascular disease. Circulation 1995; 91: 541–8
Murry CE, Kay MA, Bartosek T, et al. Muscle differentiation during repair of myocardial necrosis in rats via gene transfer with MyoD. J Clin Invest 1996; 98: 2209–17
Prentice H, Kloner RA, Prigozy T, et al. Tissue restricted gene expression assayed by direct DNA injection into cardiac and skeletal muscle. J Mol Cell Cardiol 1994; 26: 1393–401
Guzman RJ, Lemarchand P, Crystal RG, et al. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res 1993; 73: 1202–7
Kirshenbaum LA, MacLellan WR, Mazur W, et al. Highly efficient gene transfer into adult ventricular myocytes by recombinant adenovirus. J Clin Invest 1993; 92: 381–7
Quinones MJ, Leor J, Kloner RA, et al. Avoidance of immune response prolongs expression of genes delivered to the adult rat myocardium by replication-defective adenovirus. Circulation 1996; 94: 1394–401
Svensson EC, Marshall DJ, Woodard K, et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation 1999; 99: 201–5
Sakoda T, Kasahara N, Hamamori Y, et al. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol 1999; 31: 2037–47
Aoki M, Morishita R, Muraishi A, et al. Efficient in vivo gene transfer into the heart in the rat myocardial infarction model using the HVJ (Hemagglutinating Virus of Japan)— liposome method. J Mol Cell Cardiol 1997; 29: 949–59
Leor J, Quinones MJ, Patterson M, et al. Adenovirus-mediated gene transfer into infarcted myocardium: feasibility, timing, and location of expression. J Mol Cell Cardiol 1996; 28: 2057–67
Prentice H, Kloner RA, Li Y, et al. Ischemic/reperfused myocardium can express recombinant protein following direct DNA or retroviral injection. J Mol Cell Cardiol 1996; 28: 133–40
Schwarz ER, Speakman MT, Patterson M, et al. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat — angiogenesis and angioma formation. J Am Coll Cardiol 2000; 35: 1323–30
Lee LY, Patel SR, Hackett NR, et al. Focal angiogen therapy using intramyocardial delivery of an adenovirus vector coding for vascular endothelial growth factor 121. Ann Thorac Surg 2000; 69: 14–23; discussion 23-4
Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation 1999; 100: 468–74
Floyd Jr SS, Clemens PR, Ontell MR, et al. Ex vivo gene transfer using adenovirus-mediated full-length dystrophin delivery to dystrophic muscles. Gene Ther 1998; 5: 19–30
Etzion S, Barbasti IM, Granot Y, et al. Gene-delivery to the infarcted myocardium with ex-vivo modified cardiomyoblasts is superior to direct adenovirusmediated gene transfer. Card Vasc Regen. In press
Lattanzi L, Salvatori G, Coletta M, et al. High efficiency myogenic conversion of human fibroblasts by adenoviral vector-mediated MyoD gene transfer. An alternative strategy for ex vivo gene therapy of primary myopathies. J Clin Invest 1998; 101: 2119–8
Tam SK, Gu W, Nadal-Ginard B. Molecular cardiomyoplasty: potential cardiac gene therapy for chronic heart failure. J Thorac Cardiovasc Surg 1995; 109: 918–23; discussion 923-4
Robinson SW, Cho PW, Levitsky HI, et al. Arterial delivery of genetically labelled skeletal myoblasts to the murine heart: long-term survival and phenotypic modification of implanted myoblasts. Cell Transplant 1996; 5: 77–91
James J, Robbins J. Molecular remodeling of cardiac contractile function. Am J Physiol 1997; 273: H2105–18
Nakao K, Minobe W, Roden R, et al. Myosin heavy chain gene expression in human heart failure. J Clin Invest 1997; 100: 2362–70
Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res 1996; 79: 1059–63
Jones WK, Grupp IL, Doetschman T, et al. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J Clin Invest 1996; 98: 1906–7
Luo W, Grupp IL, Harrer J, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res 1994; 75: 401–9
Hajjar RJ, del Monte F, Matsui T, et al. Prospects for gene therapy for heart failure. Circ Res 2000; 86: 616–21
Hajjar RJ, Schmidt U, Kang JX, et al. Adenoviral gene transfer of phospholamban in isolated rat cardiomyocytes. Rescue effects by concomitant gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circ Res 1997; 81: 145–53
Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res 1998; 83: 15–26
Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res 1998; 83: 1–14
Beltrami P, Urbanek K, Kajstura J, et al. Evidence that huamn cardiacmyocytes divide after myocardial infarction. N Engl J Med 2001; 344: 1750–7
MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol 2000; 62: 289–319
Schneider MD. Myocardial infarction as a problem of growth control: cell cycle therapy for cardiac myocytes?. J Card Fail 1996; 2: 259–63
Williams RS. Cell cycle control in the terminally differentiated myocyte. A platform for myocardial repair?. Cardiol Clin 1998; 16: 739–54
Li JM, Brooks G. Cell cycle regulatory molecules (cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors) and the cardiovascular system; potential targets for therapy?. Eur Heart J 1999; 20: 406–20
Liu Y, Kitsis RN. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J Cell Biol 1996; 133: 325–4
Kirshenbaum LA, Schneider MD. Adenovirus E1A represses cardiac gene transcription and reactivates DNA synthesis in ventricular myocytes, via alternative pocket protein- and p300-binding domains. J Biol Chem 1995; 270: 7791–4
Kirshenbaum LA, Abdellatif M, Chakraborty S, et al. Human E2F-1 reactivates cell cycle progression in ventricular myocytes and represses cardiac gene transcription. Dev Biol 1996; 179: 402–11
Agah R, Kirshenbaum LA, Abdellatif M, et al. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53- independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest 1997; 100: 2722–8
Jackson T, Allard MF, Sreenan CM, et al. Transgenic animals as a tool for studying the effect of the c-myc proto-oncogene on cardiac development. Mol Cell Biochem 1991; 104: 15–9
Jackson T, Allard MF, Sreenan CM, et al. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol Cell Biol 1990; 10: 3709–16
Soonpaa MH, Koh GY, Pajak L, et al. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest 1997; 99: 2644–54
Poolman RA, Li JM, Durand B, et al. Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27KIP1 knockout mice. Circ Res 1999; 85: 117–27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etzion, S., Kedes, L.H., Kloner, R.A. et al. Myocardial Regeneration. Am J Cordiovosc Drugs 1, 233–244 (2001). https://doi.org/10.2165/00129784-200101040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129784-200101040-00002