Abstract
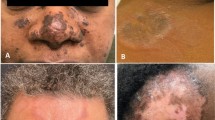
Skin involvement occurs in a third of patients with sarcoidosis. The type of lesions can range from the transient erythema nodosum to the chronic facial lesion lupus pernio. For some patients with sarcoidosis, lesions on the face or elsewhere on the body may be the major or only indication for therapy. These lesions are often chronic and the use of corticosteroids may lead to more long-term complications. Conventional alternatives to corticosteroids include antimalarial agents, methotrexate, and azathioprine. Recently, several drugs have been studied for chronic cutaneous sarcoidosis; thalidomide has been the most widely used. Thalidomide has been demonstrated to suppress tumor necrosis factor (TNF) release, which may be important at both the initial and chronic phases of the inflammation of sarcoidosis and appears to be crucial as part of the initial granulomatous response. Thalidomide has a different toxicity profile than corticosteroids or immunosuppressives. The usual dosage has recently been investigated in a dose-escalation trial, with the majority of patients responding to 100 mg/day. Drug toxicity has been reported in the sarcoidosis trials. The most serious adverse effect has been peripheral neuropathy, which often resolves by reducing the dose or discontinuing the medication. Other drugs that have been studied for sarcoidosis include infliximab and tetracyclines. Infliximab is a chimeric monoclonal antibody against TNF, and several published reports have shown it to be effective for the treatment of cutaneous sarcoidosis. The efficacy of tetracyclines for cutaneous sarcoidosis could be on the basis of their immunologic properties. In addition, these drugs have potent antimicrobial activity against Propionibacterium acnes; there is increasing evidence to suggest this may be one of the causes of sarcoidosis. However, most of the newer agents for cutaneous sarcoidosis have only been studied in small series. Over the next few years, it is hoped that there will be clinical trials to determine the role of each new therapy in the treatment of cutaneous sarcoidosis.









Similar content being viewed by others
References
Mana J, Gomez VC, Montero A, et al. Lofgren!!!2019;s syndrome revisited: a study of 186 patients. Am J Med 1999; 107: 240–5
Spiteri MA, Matthey F, Gordon T, et al. Lupus pernio: a clinico-radiological study of thirty-five cases. Br J Dermatol 1985; 112: 315–22
Baughman RP, Judson MA, Teirstein AS, et al. Thalidomide for chronic sarcoidosis. Chest 2002; 122: 227–32
Turner GA, Lower EE, Corser BC, et al. Sleep apnea in sarcoidosis. Sarcoidosis 1997; 14: 61–4
Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med 1983; 208: 525–33
Zeitlin JF, Tami TA, Baughman R, et al. Nasal and sinus manifestations of sarcoidosis. Am J Rhinol 2000; 14: 157–61
Rizzato G, Montemurro L. Reversibility of exogenous corticosteroid-induced bone loss. Eur Respir J 1993; 6: 116–9
Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis: American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999; 16: 149–73
Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995; 155: 846–51
Siltzbach LE, Teirstein AS. Chloroquine therapy in 43 patients with intrathoracic and cutaneous sarcoidosis. Acta Med Scand 1964; 425: 302S-8S
Zie J, Horowitz D, Arzubiaga C, et al. Treatment of cutaneous sarcoidosis with chloroquine: review of the literature. Arch Dermatol 1991; 127: 1034–40
British Tuberculosis Association. Chloroquine in the treatment of sarcoidosis. Tubercle 1967; 48: 257–72
Jones E, Cagen JP. Hydroxychloroquine is effective therapy for control of cutaneous sarcoidal granulomas. Am Acad Dermatol 1990; 23: 487–90
Webster GF, Razsi LK, Sanchez M, et al. Weekly low-dose methotrexate therapy for cutaneous sarcoidosis. J Am Acad Dermatol 1991; 24: 451–4
Paramothayan S, Jones PW. Corticosteroid therapy in pulmonary sarcoidosis: a systematic review. JAMA 2002; 287: 1301–7
Baltzan M, Mehta S, Kirkham TH, et al. Randomized trial of prolonged chloroquine therapy in advanced pulmonary sarcoidosis. Am J Respir Crit Care Med 1999; 160: 192–7
Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis 2000; 17: 60–6
Muller-Quernheim J, Kienast K, Held M, et al. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J 1999; 14: 1117–22
Baughman RP, Lower EE. Infliximab for refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2001; 18: 70–4
Mallbris L, Ljungberg A, Hedblad MA, et al. Progressive cutaneous sarcoidosis responding to anti-tumor necrosis factor-alpha therapy. J Am Acad Dermatol 2003; 48: 290–3
Bachelez H, Senet P, Cadranel J, et al. The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatol 2001; 137: 69–73
Nowack U, Gambichler T, Hanefeld C, et al. Successful treatment of recalcitrant cutaneous sarcoidosis with fumaric acid esters. BMC Dermatol 2002; 2 (1): 15
Kouba DJ, Mimouni D, Rencic A, et al. Mycophenolate mofetil may serve as a steroid-sparing agent for sarcoidosis. Br J Dermatol 2003; 148: 147–8
Katoh N, Mihara H, Yasuno H. Cutaneous sarcoidosis successfully treated with topical tacrolimus. Br J Dermatol 2002; 147: 154–6
Sheskin J, Convit J. Results of a double blind study of the influence of thalidomide on the lepra reaction. Int J Lepr Other Mycobact Dis 1969; 37: 135–46
Naafs B, Faber WR. Thalidomide therapy: an open trial. Int J Dermatol 1985; 24: 131–4
Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–71
Corral LG, Muller GW, Moreira AL, et al. Selection of novel analogs of thalidomide with enhanced tumor necrosis factor alpha inhibitory activity. Mol Med 1996; 2: 506–15
Kunkel SL, Lukacs NW, Strieter RM, et al. Th1 and Th2 responses regulate experimental lung granuloma development. Sarcoidosis Vasc Diffuse Lung Dis 1996; 13: 120–8
Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J 1991; 10: 4025–31
Bachwich PR, Lynch III JP, Larrick J, et al. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol 1986; 125: 421–5
Bost TW, Riches DW, Schumacher B, et al. Alveolar macrophages from patients with beryllium disease and sarcoidosis express increased levels of mRNA for tumor necrosis factor-alpha and interleukin-6 but not interleukin-1 beta. Am J Respir Cell Mol Biol 1994; 10: 506–13
Pueringer RJ, Schwartz DA, Dayton CS, et al. The relationship between alveolar macrophage TNF, IL-1, and PGE2 release, alveolitis, and disease severity in sarcoidosis. Chest 1993; 103: 832–8
Steffen M, Petersen J, Oldigs M, et al. Increased secretion of tumor necrosis factor-alpha, interleukin-1-beta, and interleukin-6 by alveolar macrophages from patients with sarcoidosis. J Allergy Clin Immunol 1993; 91: 939–49
Baughman RP, Strohofer SA, Buchsbaum J, et al. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J Lab Clin Med 1990; 115: 36–42
Baughman RP, Lower EE. The effect of corticosteroid or methotrexate therapy on lung lymphocytes and macrophages in sarcoidosis. Am Rev Respir Dis 1990; 142: 1268–71
Ziegenhagen MW, Rothe E, Zissel G, et al. Exaggerated TNF alpha release of alveolar macrophages in corticosteroid resistant sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2002; 19: 185–90
Baughman RP, Lower EE. Can persistent tumor necrosis factor release lead to refractory sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis 2002; 19: 164–6
Webster JC, Oakley RH, Jewell CM, et al. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A 2001; 98: 6865–70
Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform: expression, biochemical properties, and putative function. J Biol Chem 1996; 271: 9550–9
Tavares JL, Wangoo A, Dilworth P, et al. Thalidomide reduces tumour necrosis factor-alpha production by human alveolar macrophages. Respir Med 1997; 91: 31–9
Rowland TL, McHugh SM, Deighton J, et al. Differential regulation by thalidomide and dexamethasone of cytokine expression in human peripheral blood mononuclear cells. Immunopharmacology 1998; 40: 11–20
Moreira AL, Tsenova Berkova L, Wang J, et al. Effect of cytokine modulation by thalidomide on the granulomatous response in murine tuberculosis. Tuber Lung Dis 1997; 78: 47–55
Tramontana JM, Utaipat U, Molloy A, et al. Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med 1995; 1: 384–97
Oliver SJ, Kikuchi T, Krueger JG, et al. Thalidomide induces granuloma differentiation in sarcoid skin lesions associated with disease improvement. Clin Immunol 2002; 102: 225–36
Jacobson JM, Greenspan JS, Spritzler J, et al. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection: National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N Engl J Med 1997; 336: 1487–93
Moller DR, Forman JD, Liu MC, et al. Enhanced expression of IL-12 associated with Th 1 cytokine profiles in active pulmonary sarcoidosis. J Immunol 1996; 156: 4952–60
Moller DR, Wysocka M, Greenlee BM, et al. Inhibition of IL-12 production by thalidomide. J Immunol 1997; 159: 5157–61
Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 1999; 163: 380–6
Sheskin J. The treatment of lepra reaction in lepromatous leprosy: fifteen years!!!2019; experience with thalidomide. Int J Dermatol 1980; 19: 318–22
Carlesimo M, Giustini S, Rossi A, et al. Treatment of cutaneous and pulmonary sarcoidosis with thalidomide. J Am Acad Dermatol 1995; 32: 866–9
Lee JB, Koblenzer PS. Disfiguring cutaneous manifestation of sarcoidosis treated with thalidomide: a case report. J Am Acad Dermatol 1998; 39: 835–8
Rousseau L, Beylot-Barry M, Doutre MS, et al. Cutaneous sarcoidosis successfully treated with low doses of thalidomide. Arch Dermatol 1998; 134: 1045–6
Marriott JB, Cookson S, Carlin E, et al. A double-blind placebo-controlled phase II trial of thalidomide in asymptomatic HIV-positive patients: clinical tolerance and effect on activation markers and cytokines. AIDS Res Human Retroviruses 1997; 13: 1625–31
Ochonisky S, Verroust J, Bastuji-Garin S, et al. Thalidomide neuropathy incidence and clinico-electrophysiologic findings in 42 patients. Arch Dermatol 1994; 130: 66–9
Gerr FE, Letz R. Reliability of a widely used test of peripheral cutaneous vibration sensitivity and a comparison of two testing protocols. Br J Ind Med 1988; 45: 635–9
Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor-alpha neutralizing agent. N Engl J Med 2001; 345: 1098–104
Kavanaugh A, Clair EW, McCune WJ, et al. Chimeric anti-tumor necrosis factor-alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. J Rheumatol 2000; 27: 841–50
Moreland LW, Schiff MH, Baumgartner SW, et al. Etanercept therapy in rheumatoid arthritis: a randomized, controlled trial. Ann Intern Med 1999; 130: 478–86
Oh CJ, Das KM, Gottlieb AB. Treatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol 2000; 42: 829–30
Chaudhari U, Romano P, Mulcahy LD, et al. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 2001; 357: 1842–7
Konrad A, Seibold F. Response of cutaneous Crohn!!!2019;s disease to infliximab and methotrexate. Dig Liver Dis 2003; 35: 351–6
Zaccagna A, Bertone A, Puiatti P, et al. Anti-tumor necrosis factor alpha monoclonal antibody (infliximab) for the treatment of pyoderma gangrenosum associated with Crohn!!!2019;s disease. Eur J Dermatol 2003; 13: 258–60
Meyerle JH, Shorr A. The use of infliximab in cutaneous sarcoidosis. J Drugs Dermatol 2003; 2: 413–4
Utz JP, Limper AH, Kalra S, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest 2003; 124: 177–85
Baughman RP, Bradley DA, Raymond LA, et al. Double blind randomized trial of a tumor necrosis factor receptor antagonist (etanercept) for treatment of chronic ocular sarcoidosis [abstract]. Am J Respir Crit Care Med 2002; 165: A495
Anker SD, Coats AJ. How to recover from renaissance? The significance of the results of recover, renaissance, renewal and attach. Int J Cardiol 2002; 86: 123–30
Chung ES, Packer M, Lo KH, et al. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003; 107: 3133–40
Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003; 62: 267–9
Kloppenburg M, Verweij CL, Miltenburg AM, et al. The influence of tetracyclines on T cell activation. Clin Exp Immunol 1995; 102: 635–41
Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 2002; 40: 198–204
Ishige I, Usui Y, Takemura T, et al. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet 1999; 354: 120–3
Elkayam O, Levartovsky D, Brautbar C, et al. Clinical and immunological study of 7 patients with minocycline-induced autoimmune phenomena. Am J Med 1998; 105: 484–7
Farver DK. Minocycline-induced lupus. Ann Pharmacother 1997; 31: 1160–3
Shapiro LE, Knowles SR, Shear NH. Comparative safety of tetracycline, minocycline, and doxycycline. Arch Dermatol 1997; 133: 1224–30
Martinet Y, Pinkston P, Saltini C, et al. Evaluation of the in vitro and in vivo effects of cyclosporine on the lung T-lymphocyte alveolitis of active pulmonary sarcoidosis. Am Rev Respir Dis 1996; 138: 1242–8
Wyser CP, van Schalkwyk EM, Alheit B, et al. Treatment of progressive pulmonary sarcoidosis with cyclosporin A: a randomized controlled trial. Am J Respir Crit Care Med 1997; 156: 1571–6
Frizzell B, Stith M, Jenrette J. Management of treatment-resistant cutaneous sarcoidosis with radiation. Am J Clin Oncol 2002; 25: 573–5
Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther 1999; 1: 49–52
Grema H, Greve B, Raulin C. Scar sarcoidosis: treatment with the Q-switched ruby laser. Lasers Surg Med 2002; 30: 398–400
Acknowledgments
Dr Baughman and Dr Lower have received support for clinical trials from Celgene Corporation, manufacturers of thalidomide, and Centocor Inc., manufacturers of infliximab.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baughman, R.P., Lower, E.E. Newer Therapies for Cutaneous Sarcoidosis. Am J Clin Dermatol 5, 385–394 (2004). https://doi.org/10.2165/00128071-200405060-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00128071-200405060-00003