Abstract
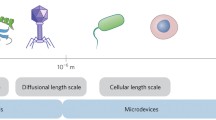
Nanotechnology, the science of creating structures, devices, and systems with a length scale of approximately 1–100 nanometers, is poised to have a revolutionary effect on biomedical research and clinical science. By operating at the same scale as most biomacromolecules, nanoscale devices can afford a detailed view of the molecules and events that drive cellular systems and that lie at the heart of disease, and thus, nanotechnology can impact the drug discovery, development, and clinical testing of novel pharmaceuticals. Already, nanoscale drug delivery vehicles are in clinical use, but those successes represent just one way in which nanotechnology will prove useful. One promising nanoscale technology under development may provide real-time, in vivo measurements of apoptosis, and thus may afford an early signal of therapeutic efficacy, both in human clinical trials and in preclinical screening. Microfluidic systems, built of nanoscale components, can enable a host of rapid, massively parallel, high-throughput screening systems, while nanoscale sensors in a wide variety of formats are ready to provide multiplexed biochemical and genetic measurements in living systems. These advances could be utilized to shave time and expense from multiple stages of the drug discovery and development effort.




Similar content being viewed by others
Notes
The use of tradenames is for product identification purposes only and does not imply endorsement
References
DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drag development costs. J Health Econ 2003; 22: 151–85
US Food and Drug Administration, Department of Health and Human Services. Innovation/stagnation: challenge and opportunity on the critical path to new medical products 2004 [online]. Available from URL: http://www.fda.gov/oc/initiatives/criticalpath/whitepaper.html [Accessed 2005 Jun 13]
National Technology Initiative. What is nanotechnology [online]? Available from URL: http://www.nano.gov/html/facts/whatIsNano.html [Accessed 2005 May 26]
Leroy BJ, Lemay SG, Kong J, et al. Electrical generation and absorption of phonons in carbon nanotubes. Nature 2004; 432: 371–4
West JL, Halas NJ. Engineered nanomaterials for biophotonics applications: improving sensing, imaging, and therapeutics. Annu Rev Biomed Eng 2003; 5: 285–92
Zhang S. Emerging biological materials through molecular self-assembly. Biotechnol Adv 2002; 20: 321–39
The Royal Society & The Royal Academy of Engineering. Nanoscience and nanotechnologies: opportunities and uncertainties 2004 [online]. Available from URL http://www.nanotec.org.uk/finalReport.htm [Accessed 2005 May 26]
Oyewumi MO, Yokel RA, Jay M, et al. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice. J Control Release 2004; 95: 613–26
Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant 1994; 14Suppl. 5: S3–7
Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin: rationale for use in solid tumours. Drags 1997; 54Suppl. 4: 15–21
Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003; 348: 2491–9
Feng SS, Mu L, Win KY, et al. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr Med Chem 2004; 11: 413–24
Gradishar WJ, Tjulandin S, Davidson N, et al. Superior efficacy of nanoparticle albumin-bound paclitaxel (abraxane, ABI-07) compared with cremophor-based paclitaxel (Taxol) in women with metastatic breast cancer: results of a phase III trial. J Clin Oncol 2005. In press
Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 2005; 5: 1–12
Kosal ME, Chou JH, Wilson SR, et al. A functional zeolite analogue assembled from metalloporphyrins. Nat Mater 2002; 1: 118–21
Giessler S, da Costa JC, Lu GQ. Hydrophobicity of templated silica xerogels for molecular sieving applications. J Nanosci Nanotechnol 2001; 1: 331–6
Bennink RJ, van den Hoff MJ, van Hemert FJ, et al. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J Nucl Med 2004; 45: 842–8
Blankenberg FG, Katsikis P, Tait J, et al. In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc Natl Acad Sci U S A 1998; 95: 6349–54
Fadok VA, Savill JS, Haslett C, et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol 1992; 149: 4029–35
Mochizuki T, Kuge Y, Zhao S, et al. Detection of apoptotic tumor response in vivo after single dose of chemotherapy with 99mTc-annexin V. J Nucl Med 2003; 44: 92–7
Schellenberger EA, Bogdanov A, Hogemann D, et al. Annexin V-CLIO: a nanoparticle for detecting apoptosis by MRI. Mol Imaging 2002; 1: 102–7
Amirghofran Z, Monabati A, Gholijani N. Androgen receptor expression in relation to apoptosis and the expression of cell cycle related proteins in prostate cancer. Pathol Oncol Res 2004; 10: 37–41
Groeger AM, Esposito V, De Luca A, et al. Prognostic value of immunohistochemical expression of p53, bax, Bcl-2 and Bcl-xL in resected non-small-cell lung cancers. Histopathology 2004; 44: 54–63
Pollard RE, Sadlowski AR, Bloch SH, et al. Contrast-assisted destruction-replenishment ultrasound for the assessment of tumor microvasculature in a rat model. Technol Cancer Res Treat 2002; 1: 459–70
Winter PM, Caruthers SD, Kassner A, et al. Molecular imaging of angiogenesis in nascent Vx-2 rabbit tumors using a novel alpha(nu)beta3-targeted nanoparticle and 1.5 tesla magnetic resonance imaging. Cancer Res 2003; 63: 5838–43
George ML, Dzik-Jurasz AS, Padhani AR, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg 2001; 88: 1628–36
Bogunia-Kubik K, Sugisaka M. From molecular biology to nanotechnology and nanomedicine. Biosystems 2002; 65: 123–38
Xu XH, Brownlow WJ, Kyriacou SV, et al. Real-time probing of membrane transport in living microbial cells using single nanoparticle optics and living cell imaging. Biochemistry 2004; 43: 10400–13
Geho DH, Lahar N, Ferrari M, et al. Opportunities for nanotechnology-based innovation in tissue proteomics. Biomed Microdevices 2004; 6: 231–9
Cui Y, Wei Q, Park H, et al. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001; 293: 1289–92
Bunimovich YL, Ge G, Beverly KC, et al. Electrochemically programmed, spatially selective biofunctionalization of silicon wires. Langmuir 2004; 20: 10630–8
Patolsky F, Zheng G, Hayden O, et al. Electrical detection of single viruses. Proc Natl Acad Sci U S A 2004; 101: 14017–22
Alvarez M, Carrascosa LG, Moreno M, et al. Nanomechanics of the formation of DNA self-assembled monolayers and hybridization on microcantilevers. Langmuir 2004; 20: 9663–8
Emerson RJ, Camesano TA. Nanoscale investigation of pathogenic microbial adhesion to a biomaterial. Appl Environ Microbiol 2004; 70: 6012–22
Zhang Y, Venkatachalan SP, Xu H, et al. Micromechanical measurement of membrane receptor binding for label-free drug discovery. Biosens Bioelectron 2004; 19: 1473–8
Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol 2003; 21: 41–6
Wu X, Bruchez MP. Labeling cellular targets with semiconductor quantum dot conjugates. Methods Cell Biol 2004; 75: 171–83
Hirsch LR, Jackson JB, Lee A, et al. A whole blood immunoassay using gold nanoshells. Anal Chem 2003; 75: 2377–81
Chen RJ, Bangsaruntip S, Drouvalakis KA, et al. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc Natl Acad Sci U S A 2003; 100: 4984–9
Thorsen TA. Microfluidic tools for high-throughput screening. Biotechniques 2004; 36: 197–9
Puckett LG, Dikici E, Lai S, et al. Investigation into the applicability of the centrifugal microfluidics platform for the development of protein-ligand binding assays incorporating enhanced green fluorescent protein as a fluorescent reporter. Anal Chem 2004; 76: 7263–8
Khademhosseini A, Yeh J, Jon S, et al. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip 2004; 4: 425–30
Sinclair J, Pihl J, Olofsson J, et al. A cell-based bar code reader for high-throughput screening of ion channel-ligand interactions. Anal Chem 2002; 74: 6133–8
National Cancer Institute. NCI Alliance for Nanotechnology in Cancer [online]. Available from URL: http://nano.cancer.gov [Accessed 2005 May 26]
Acknowledgments
The authors wish to acknowledge the contributions of Joe Alper and Travis Earles in preparing this manuscript.
Preparation of this article was supported by the National Cancer Institute. The authors have no conflicts of interest directly relevant to the contents of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrari, M., Downing, G. Medical Nanotechnology. BioDrugs 19, 203–210 (2005). https://doi.org/10.2165/00063030-200519040-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200519040-00001