Abstract
Extremely low birth-weight newborns (<1000g) experience low levels of thyroid hormone that vary inversely with the severity of neonatal illness and the extent of developmental immaturity with levels reaching a nadir at ≈7 days after birth; this phenomenon can persist for several weeks. In the absence of transplacental passage, 30–50% of these neonates cannot generate sufficient quantities of thyroid hormone to meet postnatal demands, placing them at an increased risk for developmental delay and cerebral palsy. Population surveys and interventional trials suggest that a therapeutic opening exists during a ‘window of opportunity’ corresponding to this period of diminished capacity. Variables to consider before intervention focus on the consideration that supplementation of both the substrate thyroxine and the active hormone triiodothyronine may be necessary in quantities that do not suppress thyroid-stimulating hormone release, yet overcome the persistence of increased conversion to 3,3′5′-triodo-L-thyronine, terminal deiodination, and activity of the sulfation inactivation pathways, as well as the diminished capacity of the newborn to accommodate postnatal physiologic changes. Single daily replacement doses may suppress levels of converting enzymes in the brain, suggesting that physiologic ‘mimicry’ provided by a constant infusion may be the preferred dosing option. Properly powered clinical trials targeting long-term developmental outcomes are needed to discern whether these interventions will do more than simply elevate blood levels of thyroid hormones to the target values of either the fetus or developing neonate. Identifying the appropriate indications for supplementation may alleviate individual pain and distress due to disability for several hundred extremely low birth-weight neonates each year in the US alone, and save society a pro-rated lifetime cost of nearly $US1 million per child.
Similar content being viewed by others
In the US, despite extraordinary advancements, the rate of premature birth has increased by 13% since 1992, at a current healthcare cost of nearly $US14 billion annually, representing almost 50% of all healthcare dollars spent on individuals during the newborn period.[1] Impressively, the overall percentage survival of extremely low birth-weight (ELBW; <1000g) neonates has continuously increased and now neonates that have completed <24 postconceptional weeks of gestation survive on a regular basis, and this is associated with an increase in the absolute number of tiny patients with a high risk of long-term disability.[2] More specifically, of the ≈9000–20 000 ELBW neonates born each year in the US (0.25–0.5% of ≈4 million births), about 15% will develop cerebral palsy, accruing an additional pro-rated lifetime cost for management of ≈$US1 million per child.[3–5] Thus, the personal tragedy of survival with disability, as well as the continuing economic impact on society, is substantial.
The pathogenesis of the brain damage in ELBW neonates is multifactorial and includes nutritional, metabolic, infectious, developmental, and hormonal factors. Undernutrition, malnutrition, respiratory distress syndrome, hypoxia, sepsis, cardiac anomalies, intraventricular hemorrhage, gastrointestinal disorders, and surgery are common morbidities in these infants, and require sophisticated and intensive care.[6–8] A number of authors have reported increased morbidity and mortality associated with low neonatal serum thyroxine (T4; L-3,5,3′,5′-tetraiodothyronine) levels in preterm neonates, and recent studies have documented low T4 and free T4, or low triiodothyronine (T3; 3,5,3′-triiodothyronine) levels in association with the neonatal morbidity.[3,6,9–14] Untreated thyroid hormone deficiency in utero or during infancy is known to result in neurologic sequelae and mental retardation.[15–17] Cord blood levels of TBG, total and free T4, and T3 are lower in premature infants. Their TSH surge is blunted, and thyroid hormone levels drop precipitously to reach a nadir at about 1 week of postnatal age. Circulating levels of T4 and TSH are lower than in full-term infants during the first 2 weeks of life.[4,6,18–20] Whether transient hypothyroxinemia is the cause or an effect of the neonatal morbidity and whether thyroid hormone deficiency contributes to the brain damage observed in ELBW neonates remain unclear. Severe illness in adults is often associated with low T3 and T4 levels and a normal or low serum thyroid-stimulating hormone (TSH) level, a metabolic condition that is referred to as the low T3 syndrome, the euthyroid sick syndrome, or the non-thyroidal illness syndrome.[17,21,22] A marked reduction in the T4 level to <4 μg/dL (<52 nmol/L) in such patients is associated with mortality of 50% and a level <2 μg/dL (26 nmol/L) is associated with mortality that approaches 80%.[17] Whether the euthyroid sick syndrome/non-thyroidal illness syndrome represents a teleologic adaptation to severe pathophysiologic insult or a pathophysiologic state needing correction remains controversial.
The management of transient hypothyroxinemia of prematurity (THOP) also remains controversial. The definition of THOP, with regard to the T4 level for diagnosis, is unclear. However, there is a growing convergence of adult and pediatric data around a threshold value for T4 of <4 μg/dL (<52 nmol/L),[17,23] consistent with observations of an association between such levels and the occurrence of morbidity in neonates (table I).
To date, there have been seven trials of treatment with supplemental T4 or T3 in neonates with THOP, which have used various regimens and provided conflicting results (table II).[24–32] The decreased postnatal surge in TSH levels in ELBW neonates, their nadir in thyroid hormone levels at ≈7 days, the persistence of the low levels of these hormones during an illness that lasts up to 4 weeks, and the high level of inactivation of T3 for weeks after birth are each reasons why therapy was commenced at birth in each of these trials and, in some, continued for 4–6 weeks. None of the THOP literature cited in table II screened patients first and then initiated drug treatment in response to low thyroid hormone levels. Interestingly, criteria for such an approach have been suggested, using a statistical norm of within −1 standard deviation of the cord T4 levels adjusted for weight in neonates of an equivalent gestational age.[33] This does not correlate with long-term outcomes, does not address the immediate needs of T3, and will not resolve deficiencies in thyroid hormone actions in the first postnatal week of life.
In the largest and only controlled long-term follow-up study to date, van Wassenaer et al.[29] also noted an 18-point improvement in Bayley scores of mental development at 2 years of age following levothyroxine (T4) treatment in infants born before 27 weeks of gestation, with no difference in behavioral scores versus placebo. In infants in the >27-week gestational age group, behavioral problems following treatment, as well as a ten-point lower Bayley score versus placebo, were seen at 2 years of age and persisted at 5 years of age (although both groups had Bayley scores that were within the normal range). Significantly, the advantage in favor of treatment in the <27-week gestational age group included an improvement in the neuromotor examination.[34,35] This would appear to be a definitive result and was confirmed by subsequent IQ assessments that showed worse developmental outcomes at ≈6 years of age only in the ≥29-week gestational age individuals;[34] however, the subgroup analysis was too small to provide sufficient power to make any conclusions (at age 2 years, there were only 18 infants with behavioral problems). Moreover, the results were not thought to be an immediate consequence of the neonatal levothyroxine treatment, as no significant differences in neonatal free T4 levels existed between children with and without behavioral problems,[35] while at 5 years of age better outcomes were found in the infants with the highest free T4 levels.[36] Another limitation of this study was that the subgroup analysis of neonates of <27 weeks’ gestation was not pre-specified or stratified in the trial’s randomization. In addition, there were unbalanced numbers between treatment groups (levothyroxine group: 18 infants of 25 or 26 weeks gestational age and 17 of 27 weeks; placebo group: 18 infants of 25 or 26 weeks’ gestational age and 13 of 27 weeks) in that subgroup. Nevertheless, this study did tocus on ELBW neonates as the group with a well defined developmental ‘window of vulnerability’ in need of further study. The effect of levothyroxine treatment on lowering the occurrence of cerebral palsy and developmental delay appears substantial in studied individuals who were born between 25 and 28 weeks of gestation.
The series of studies and follow-up reports also emphasizes the theoretical risk of excessive levothyroxine supplementation in any age group. In tact, in comparison to perinatal interventions at the same gestation, there is some concern that in utero thyrotropin-releasing hormone (TRH) exposure (see section 1.2) actually delays development and may even suppress the hypothalamic-pituitary axis[37,38] while, in contrast, antepartum interventions tor maternal hypothyroidism are especially important tor the fetus in the first trimester.[14,39] Osborne[30] published a detailed meta-analysis of these studies, and concluded that there was no conclusive evidence that the use of thyroid hormone supplementation in preterm babies was effective in reducing the risk of morbidity caused by insufficient thyroid hormone levels. Clearly, additional well designed clinical trials are needed to determine whether thyroid hormone replacement can actually improve outcomes and lower the occurrence of cerebral palsy in premature neonates.
In the preterm neonate, postnatal adaptations in thyroid function are superimposed upon an immature hypothalamic-pituitary axis and withdrawal trom a still-active placenta (a source of TRH), as well as interruption of the maternal source of thyroid hormones.[14,39] Not only are cord blood levels of thyroxine-binding globulin (TBG), total and tree T4, and T3 lower in premature neonates, but the neonatal TSH surge is blunted, and thyroid hormone levels drop to a nadir at about 1 week after birth.[18,40–44] Additionally, there are lower activities of tissue type I (predominantly found in the liver) and type II (predominantly found in the brain) monodeiodinase, presumably leading to lower rates of peripheral conversion of T4 to T3, and higher levels of monodeiodinase-III (creates inactive 3,3′5′-triodo-L-thyronine [rT3]) in the placenta and liver compared with those found in adult tissues.[45]
Lower TSH levels are also found in preterm newborns, along with higher levels of inactive iodothyronines, including rT3 and the sultated derivatives of T3 and T4.[43] Total plasma T4 levels are low as a result of decreased synthesis, increased metabolic clearance, and/or reduced T4 binding by TBG, the major carrier protein tor this hormone.[43] The decreased TBG levels and binding appear to be due to neonatal morbidities, and the administration of intravenous nutritional substrates.[6–8] Measurements of tree T4 levels vary directly according to gestational age and may or may not be low during the first week of lite, relative to intrauterine values.[6–8]
Available data suggest that ELBW premature neonates (23–27 weeks’ gestational age) are the group at highest risk of development delay and most likely to benefit trom thyroid hormone supplementation.[29] It is necessary to emphasize that the concept tor intervention in THOP is most consistent with the notion of supplementing an otherwise normal hormonal system (albeit, developmentally immature and further impaired by acute illness) rather than providing therapy tor a recognized disease process, as in congenital hypothyroidism.
In view of the absolute requirement tor thyroid hormone in normal brain development in utero, as well as the recognized critical window of vulnerability tor the ELBW neonate, it would appear obvious to treat this group of infants. However, there are many other biologic and pragmatic considerations, including the tact that excessive thyroid hormone levels can also be detrimental.[15,16] This article attempts to cross many categoric boundaries of applied human physiology to highlight the strengths, weaknesses, risks, and benefits of considering intervention or withholding treatment with thyroid hormone in neonates with THOP.
1. Confounding Clinical Considerations
In ELBW premature neonates, most organ systems are immature and the requirement tor and responsiveness to thyroid hormone are unclear. Thus, consideration of the effects of thyroid hormone on tissues other than those of the CNS is necessary.
1.1 Cardiovascular Effects
Sympathomimetics and thyroid hormones lead to similar effects on the heart, inducing tachycardia and increasing the force and velocity of contraction.[46] Extranuclear effects of T3 include an increase in protein synthesis, primarily through the transport of amino acids, sugars, and calcium across the myocyte membrane.[46] These non-genomic effects include actions on other cell organelles and occur independently of the cognate thyroid hormone receptor family.[46,47] Thyroid hormone receptor effects are mediated via transcription of thyroid-responsive genes. For example, T3 can increase the speed of diastolic relaxation via induction of the calcium adenosine triphosphatase enzyme of the sarcoplasmic reticulum, leading to more efficient pumping.[46]
In general, thyroid hormone supplementation increases cardiac output by decreasing peripheral arterial vascular resistance and increasing venous tone which, together, result in increased blood return to the heart without altering baseline blood pressure.[46] Each of these effects could be beneticial in ELBW neonates at risk of impaired oxygen delivery. In adults, although thyroid hormone therapy is associated with atrial arrhythmias (ventricular arrhythmias are rare) and emboli, these complications are not commonly reported in neonates, even in those with neonatal Graves disease secondary to transplacental passage of TSH-receptor activating autoantibodies.[8,10]
When neonates of <28 weeks’ gestational age receive levothyroxine treatment, the mean daily heart rate fails to show the gradual slowing typical of the first 2 postnatal weeks.[48] In addition, after a single dose of liothyronine (T3) in combination with a subsequent daily dose of levothyroxine, no effects, compared with placebo, were noted on either the mean daily heart rate or mean arterial blood pressure.[32]
After cardiac surgery in infants and children, T3 levels fall by 60% and remain low for up to 8 days following the procedure, a period characterized by increased hormone inactivation by iodothyronine monodeiodinase-III.[49] Supplementation with liothyronine after neonatal cardiac surgery is associated with an increase in systolic blood pressure, with no changes in diastolic blood pressure or heart rate.[49] With these concerns in mind, there appear to be few cardiovascular reasons to restrict the use of supplemental thyroid hormone (until pathologically high hormone levels are achieved) and there may even prove to be beneficial effects under certain conditions, such as following myocardial ischemia and surgery for congenital heart disease, as well as in all patients who require extra-corporeal membrane oxygenator treatments.[46,49]
1.2 Gastrointestinal and Lung Maturation
More than 30 years ago, Moog[50] reviewed the beneficial effects of the thyroid and glucocorticoid hormones in maturing the epithelial cell functions of the developing gut. Indeed, it was these insights that ultimately led Liggins[51] to exploit the effects of glucocorticoids on the out-pouching of the anterior foregut, which later becomes the lung, to accelerate lung (surfactant) maturation and to subsequently use thyroid hormones for the same clinical purpose.[47,52,53]
Thyroid hormone receptors are present in the human lung at as early as 13.5 weeks’ gestation,[47] and by analogy with changes in glucocorticoid receptors, it was important to assess the impact of thyroid hormone on the developing lung. During pregnancy, weekly intra-amniotic administration of levothyroxine increased the lecithin/sphingomyelin ratio and lowered the occurrence of respiratory distress syndrome in neonates with birth weights between 1000 and 1500g, but was not proven to be helpful in neonates with birth weights below or above this range; no effects on morbidities were noted and no follow-up studies were reported.[54] Related but more extensive studies exploited the transplacental passage of maternally administered TRH to treat the fetus in utero; they too demonstrated slight benefits of treatment on lung maturation, but raised many more concerns about potential untoward effects.[37,38] Thus, while both glucocorticoids and thyroid hormones can advance lung and gut performance, unlike the effects of antenatal glucocorticoids on reducing necrotizing enterocolitis, there are no data to indicate either an advantage or disadvantage from the improvements in gut functions following thyroid hormone supplementation in newborns;[37,38] however, hypermotility remains a theoretical, albeit unreported, postnatal concern.
Ex utero treatment with thyroid hormone for the prevention of hyaline membrane disease, for weaning from ventilator support, or for the prevention of chronic lung disease in ELBW neonates has produced only marginal benefits, and to date there is no convincing evidence that treatment in utero with TRH or postnatal thyroid hormone supplementation reduces the severity of respiratory distress syndrome.[27,28,31,37,55] In fact, there is some concern that in utero TRH exposure actually causes delays in neurodevelopment and may even suppress the function of the hypothalamus-pituitary axis.[37,38]
1.3 Sepsis
Infection (commonly sepsis) occurs in as many as 20–40% of ELBW neonates and is associated with a hypermetabolic state, nitric oxide-dependent inappropriate vasodilation, and increased secretion of various cytokines.[56] Intuitively, supplementation with thyroid hormone may be either beneficial by increasing oxygen delivery and maintaining sympathetic vascular tone or it can conceivably create a negative synergy by further increasing oxygen consumption beyond the critical oxygen point through its cardiovascular and metabolic effects.[57,58] TSH suppression during sepsis is thought to be due to acute-phase reactants (interleukin [IL]-1, tumor necrosis factor-α [TNFα], endotoxin, and IL-6), since infusion of these substances can mimic clinical changes.[15,17,56] However, IL-8, IL-10, and interferon do not produce similar effects.[17] IL-1, TNFα, and IL-6 may also alter monodeiodinase activity.[17,45,46,59] Thyroid hormone supplementation in neonates with sepsis has not been undertaken systematically.
1.4 Disease and Immaturity of the Liver and Kidneys
In newborns, iodine excretion by the immature kidneys is commonly in excess of iodine intake, creating the potential for deficiency (see section 2.2.4).[41,42] In addition, glomerular filtration of iodide is dependent on circulating iodide levels with negligible tubular resorption; fecal excretion is also negligible.[41,42] Although most circulating T3 originates trom conversion of T4 in the liver by monodeiodinase-I, there is no evidence that either liver disease or renal disease affects thyroid function in the neonate.
ELBW neonates are prone to rickets due to inadequate intakes of vitamin D, calcium, and phosphate. Thyroid hormone excess is associated with a net loss in bone density and osteopenia; however, there are no convincing data to argue that thyroid hormone replacement or supplementation alone will affect either the calcium-phosphate balance or vitamin D metabolism by the kidney or liver or increase the risk of fracture in adults or neonates.[21,60,61]
1.5 Metabolic Effects
Temperature, increased brown tat metabolism, and increased oxygen consumption are derivative effects of exposure to the extrauterine environment that are dependent, in part, on thyroid hormone.[57,58,62–64] In animals, levothyroxine supplementation can be shown to stimulate thermogenesis by brown adipose tissue and decreased thermogenesis in premature neonates is largely due to the relative immaturity of the brown adipose tissue biochemical thermogenic capacity.[57,58,62] There is little evidence to argue against treating ELBW neonates with thyroid hormone because of any potential adverse effects on the metabolic rate or due to a failure to gain weight.
2. Therapeutic Considerations
2.1 Use of Glucocorticoids
TSH release is pulsatile and varies with the glucocorticoid level; high glucocorticoid levels can suppress TSH secretion as well as pituitary responses to TRH.[8] This is a concern as antenatal administration of glucocorticoids is used to mature lung function in nearly 90% of neonates in the <28-week gestational age group who are most likely to benefit trom thyroid hormone supplementation. Moreover, the adrenal and thyroid hormone systems are physiologically and developmentally linked, and a correlative relationship has been suggested between low cortisol and low thyroid hormone levels in preterm infants, particularly the sickest preterm infants.[63–66] T4 may play a role in the metabolism of cortisol to biologically inactive cortisone,[65–68] and therefore it may be prudent to monitor cortisol levels in patients receiving thyroid hormone supplementation. In addition, cortisol plays a role in late fetal maturation of many organ systems,[51] including the maturation of hepatic monodeiodinase-I (which converts T4 to T3), and at physiologic levels enhances TSH expression in the anterior pituitary.[67]
Prenatal treatment with dexamethasone has been reported to increase cord plasma T3 levels and renal clearance of iodide, and to also be associated with lower tree T4 levels.[59,68] In contrast, postnatal data show that elevated glucocorticoid levels due to exogenous administration of these agents suppress TRH secretion and the attendant release of TSH, reduce production of T4 and, due to reduced monodeiodinase-I levels, reduce conversion of T4 to T3, and increase thyroid hormone inactivation via elevation of monodeiodinase-III enzyme activity (particularly in conditions of low oxygen delivery) culminating in a net suppression of the entire hypothalamic-pituitary-thyroid axis.[7,8,59,68]
In their clinical trial, Biswas et al.[55] reported that the best clinical outcomes were achieved when patients had cortisol levels in the mid-range during postnatal thyroid hormone plus hydrocortisone supplementation and that worse pulmonary outcomes were achieved in association with cortisol levels that were within either the highest or lowest 20th percentiles. Valerio et al.[32] did not tind differences in endogenous cortisol levels between neonates who received levothyroxine and/or liothyronine supplementation versus those given placebo; however, the groups studied were small. It would appear prudent, therefore, to consider that excessive or prolonged use of exogenous glucocorticoids may result in suppression of blood thyroid hormone levels in a maturational age- and disease-specific fashion.
2.2 Nutrients and Other Drugs
2.2.1 Dopamine
Dopamine directly inhibits TSH secretion and suppresses T4 levels in adults and may show similar effects in premature neonates with secondary reductions in thyroid hormone levels.[8,17,69] Opioid drugs can suppress TSH levels while increasing serum TBG levels, yet show no clearly recognized problems in relation to neonates.[8,69]
2.2.2 Heparin
Heparin (a known activator of lipoprotein lipase) is commonly used to maintain the patency of arterial catheters (e.g. 0.5 U/mL). Heparin, alone or in combination with infused triglycerides, may elevate tree tatty acid (FFA) levels.[17,69] Triglyceride emulsions are routinely used as a source of parenteral tat and FFA in preterm infants in the first weeks after birth. Levels of FFA >2–5 mmol/L, can dissociate T4 bound to already low levels of albumin or TBG.[17,22,69,70] This may explain why tree T4 levels may appear sufficient in some preterm infants with low T4 levels.
2.2.3 Soy Proteins
In rats, soy proteins increase hepatic thyroid hormone receptor-β1 expression but inhibit thyroid hormone binding to DNA.[71] Infants with congenital hypothyroidism fed soy formula show a prolonged increase in TSH and require higher doses of levothyroxine to normalize serum T4 levels.[72] In preterm neonates, soy-based formulas are also typically avoided because they have other nutritional inadequacies.
2.2.4 Iodine
Iodine balance during pregnancy can affect maternal, fetal, and subsequently, neonatal thyroid function, and thereby, long-term neurodevelopment.[39,73] Maternal iodine intake is gradually diminishing in the US (recommended intake of 200 μg/day in pregnant women), presumably because of worry about (iodide-supplemented) salt intake, and this has raised public health concerns about a possible reemergence of maternal iodine deficiency and accompanying hypothyroxinemia.[74,75] Background levels of iodine in maternal diets also vary among countries.[42] Recent data suggest that hypothyroxinemia during pregnancy, without subclinical or clinically apparent maternal hypothyroidism, is associated with a lowered IQ in the child, and this has raised additional concerns regarding iodine deficiency in premature infants.[15,16,39,73,76,77] During pregnancy, iodine deficiency is associated with a decrease in fetal head growth if iodine supplementation is initiated too late.[73]
ELBW premature neonates have a negative iodine balance during the early postnatal weeks.[42,78] Unlike term infants, premature neonates seem unable to adapt to the extrauterine environment by augmenting thyroidal iodine uptake and increasing T4 secretion.[19,42,78,79] Levels of iodine in the breast milk or in conventional formula (both contain ≈25μg per ounce [≈80μg/100mg]) are typically too low to fulfill the high requirements of premature neonates, but were increased in preterm formula and breast milk fortifiers after two reports by Ares et al.[42,80] The target iodine intake in neonates is 30 μg/kg/day.[42,80]
Iodinated Compounds
Iodinated compounds such as disinfectants (e.g. betadine or povidone; iodine 10 000 μg/mL), amiodarone, and intravenous contrast agents (iodine 300 000 μg/mL) can produce iodine overload,[69] with the attendant transient neonatal hypothyroidal response,[81] through a mechanism known as the Wolff-Chaikoff effect.[82] Excessive exposure to free iodine inhibits organic binding by the normal thyroid gland creating an inhibition of thyroid hormone synthesis.[82] Chlorhexidine skin cleansers should be used, instead of other skin cleansers, as they are not absorbed, and have no known organ toxicity except skin irritation on prolonged contact, a problem that is especially important for neonates with poorly keratinized skin (i.e. those in the 400–600g weight range).[83]
3. Conceptual and Pragmatic Considerations for Treatment
3.1 What is a ‘Normal’ Thyroid Hormone Level for an Extremely Low Birth-Weight Neonate?
‘Normal’ thyroid hormone levels in human neonates have been ‘operationally’ defined by examining the correlation of neonatal plasma thyroid hormone levels with long-term neurodevelopment outcomes. Specifically, in THOP and other conditions associated with low thyroid hormone levels, an impaired long-term neurodevelopmental outcome appears to be the most useful and important marker (table I).[84]
No short-term, co-morbid, or concurrent physiologic measures are available to assess the efficacy of thyroid hormone supplementation, including its effects on neuroendocrine function; growth hormone, prolactin, and insulin-like growth factor-I levels appear to be unaffected by levothyroxine supplementation.[24] It appears, therefore, that the best guide for directing therapy would be the titration of dosages in order to achieve neonatal plasma levels for several components of the thyroid hormone axis that are similar to those of more mature, healthy full-term newborns who rarely develop hypothyroxinemia and have normal neurodevelopment. This target would be a serum T4 level of 50–180 nmol/L (i.e. 4–14 μg/dL) and a free T4 level of 10–35 pmol/L (0.8–2.7 μg/dL) based primarily on our interpretation of the empirical data reviewed in tables I and II and correlative results from van Trotsenburg et al.,[85] Tilotson et al.,[23] and Rovet[84].
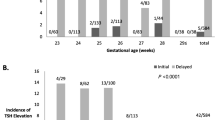
In the fetus, TSH feedback responsiveness develops at mid-gestation, along with maturation of the pituitary portal vascular system.[15,16,18,40,43,67] In each study of thyroid hormone supplementation in patients with THOP, TSH levels were markedly suppressed in ELBW neonates, to levels that are neither seen during normal development in utero nor after birth (table II). There are at least two categoric ways of achieving an ‘age-appropriate normal’ TSH level during T4 treatment. One approach would be to provide thyroid hormone as a continuous infusion (physiologic ‘mimicry’), rather than as a pharmacologic, once-daily bolus. A second approach would be to augment basal hormone levels to merely ‘supplement’ the endogenous production without suppressing the entire hypothalamus-pituitary-thyroid axis. In a recent study of levothyroxine treatment of infants with Down syndrome, TSH levels were maintained in the range of 0.4–4 mIU/L using dosages as low as a daily bolus dose of 4 μg/kg.[85] These patients achieved better motor development and showed a trend towards improved attainment of mental development milestones compared with control individuals. Ex utero target levels of 0.4–4 mIU/L seem reasonable, even though they are not as high as in utero TSH levels (figure 1).[44]
Plasma levels of (a) human free thyroxine (T4) and (b) thyroid-stimulating hormone (TSH) in premature neonates, term neonates, and fetuses by postmenstrual age (reproduced from Morreale de Escobar and Ares,[44] with permission, Copyright 1998, The Endocrine Society).
3.2 Supplementation with Levothyroxine (T4), Liothyronine (T3) or Both?
T4 functions as a substrate for conversion to the active circulating hormone T3 by type I monodeiodinase (primarily in the liver) and active local tissue T3 by monodeiodinase-II (primarily in the brain).[45,86,87] About 20% of the circulating T3 is actually secreted by the thyroid gland.[86] Studies in rats by Calvo et al.[88] have suggested that circulating T3 levels contribute marginally to cerebral intracellular T3 levels early in development and that T4 is very efficient in elevating brain levels of T3 via local conversion by monodeiodinase-II. In human neonates van Wassenaer et al.[89,90] showed that supplementation with levothyroxine alone suppresses serum T3 levels in preterm babies (presumably by suppressing TSH secretion)[48] and that a single liothyronine dose on day 1 followed by daily levothyroxine is associated with a sustained increase in T3 levels (possibly by stimulating hepatic monodeiodinase-I maturation and augmenting the conversion of T4 to T3). Unfortunately, a subsequent report from this group by Valerio et al.[32] that included control individuals who did not receive treatment, did not replicate these original observations of the effect of liothyronine. A study of 100 premature neonates showed a possible survival benefit associated with treatment with liothyronine and levothyroxine, but the levothyroxine was given at doses higher than those used in most other studies (levothyroxine 25 μg/day and liothyronine 5 μg/day).[26]
In conclusion, supplementation with both levothyroxine and liothyronine may be beneficial with regard to maturation and/or function of the extraneural tissues, such as the heart, lungs, and the gastrointestinal tract, where immediately available active thyroid hormone (T3) may be needed shortly after birth. Moreover, since increased rates of T4 inactivation to rT3 and sulfated analogs persist in premature neonates for several weeks after birth, the administration of supplemental liothyronine in ELBW neonates may be beneficial during the acute phase of their illness. However, this hypothesis, although logical, remains to be proven.
3.3 Supplemental or Therapeutic Dosing: 4 μg/kg/day versus 8 μg/kg/day?
Doses of levothyroxine that are as low as 6–8 μg/kg/day result in depression of TSH levels in ELBW neonates (table II). In thyroidectomized adult rats, the use of levothyroxine plus liothyronine (at levels that do not suppress TSH) resulted in a 50% reduction in the dosage of T4 necessary to provide a euthyroid phenotypic status as well as normal intracellular levels of T3.[91] In view of this, if one were to extrapolate data from hormone turnover studies in a 70kg adult (with a normal thyroid hormone production rate) in order to determine the doses of both levothyroxine and liothyronine required by a neonate (based on a pro-rated body surface area), treatment interventions would require ≈5–6 μg/kg/day of levothyroxine and 0.3–0.4 μg/kg/day of liothyronine.[91] Due to the immature pattern of conversion of T4 to T3 in ELBW premature neonates that is associated with the postnatal delay in the induction of monodeiodinase-I and increased conversion of T4 to inactive metabolites (i.e. due to high levels of monodeiodinase-III), an initial liothyronine dose of 1 μg/kg/day is a reasonable suggestion when used in combination with a reduced dose of levothyroxine in the range of 4–6 μg/kg/day, which may achieve a normalized blood TSH level.[32,92] A 4 μg/kg/day dosage may not have as great an inhibitory effect on endogenous thyroid hormone production via suppression of endogenous TRH and TSH compared with an 8 μg/kg/day dosage,[85] but at this time no data are available to further resolve the issue.
3.4 Continuous Infusion or Once-Daily Bolus?
The original report by van Wassenaer et al.[29] showed a likely neurodevelopmental benefit of once-daily levothyroxine therapy in neonates of <28 weeks’ gestation; however, this is not how the endogenous hormone is released and presents an intriguing pharmacologic and biologic dilemma. It can be demonstrated in rats that the activity of brain monodeiodinase-II is inversely regulated by T4, so that when the supply of T4 falls there is a compensatory increase in enzymatic conversion of T4 to T3, resulting in sustained intraneuronal T3 levels.[88] In humans, brain monodeiodinase levels also correlate with tissue T3 levels.[87] Thus, using a bolus dosing regimen, a secondary downregulation of enzymatic conversion of T4 to T3 might result along with a substrate-induced reduction in brain monodeiodinase-II activity that would likely persist beyond the fall of the transiently elevated plasma T4 level.[87,93] In addition, in sick neonates, low levels of TBG may accentuate the suppression scenario since the resulting daily peak in plasma T4 levels can, in principle, saturate available circulating T4 protein-binding sites, transiently increasing free T4 levels, and paradoxically lowering intraneuronal levels of T3 by suppressing monodeiodinase-II.[93] Additionally, the effective half-life of T4 in term newborns is ≈3.6 days, somewhat reduced compared with that in adults.[93,94] This would also exaggerate the fluctuations in blood levels of T4 following a bolus dose, especially when given intravenously, due to its immediate access to the vascular tree. A shortened T3 half-life in premature neonates was also estimated by van Wassenaer et al.[90] and was confirmed more recently by Biswas et al.[55] and Cools et al.[92]
Taken together, a lower daily per kilogram dosage of levothyroxine and a sustained rate of its infusion may better ‘mimic’ the endogenous physiologic thyroid hormone production rates and result in a lesser degree of TSH suppression. Since most ELBW neonates are not able to receive full enteral fluids until 10–21 days after birth[56] and prior to that point a continuous gavage infusion is common, combining the continuous infusion of thyroid hormone with the gavage infusion will solve the pragmatic problem of an additional continuous intravenous administration. Interestingly, neither the administration of a continuous infusion nor that of low supplemental doses (e.g. 4 μg/kg/day) have ever been tested in ELBW neonates; therefore, more empirically derived evidence is needed.
3.5 What Gestational Age and What Duration of Therapy for Transient Hypothyroxinemia of Prematurity?
Levothyroxine supplementation studies to date have provided data on thyroid hormone levels through 42 postnatal days (table II). Smaller and sicker infants demonstrate a longer duration of low thyroid hormone levels and a greater postnatal fall in circulating thyroid hormone levels. TSH levels typically remained unchanged for nearly 4 postnatal weeks.[7,8,18,44,93] Since ELBW neonates (typically with <28 complete weeks of gestation) may be ill for several weeks after birth, it appears from the available data that thyroid hormone supplementation for 4–6 weeks would be appropriate to entirely encompass the period of maximal vulnerability, followed by a tapering phase where dosages are modified according to effects on the thyroid hormone axis as seen upon sequential blood testing. This is our interpretation and empiric recommendation for the duration of thyroid hormone therapy based on the observational information in tables I and II and other published literature.[7,8,18,44,93]
3.6 Should Iodide Be Supplemented?
Interruption of the maternal supply of iodine to the neonate necessitates supplementation of iodide levels from exogenous sources; supplementation is generally considered adequate at about 30 μg/kg/day, based on fetal thyroid accretion rates.[41,42,74,78,95] ELBW neonates often have impaired thyroid hormone synthesis due to limited iodide availability and variable gut absorption of iodide specifically and other nutrients in general.[41,42,56,78] Monitoring neonatal intake from all sources will allow studies of iodide balance since fecal excretion is negligible and glomerular filtration is dependent on circulating iodide levels with minimal tubular reabsorption.[41,42,78] Maternal background levels of iodide may also vary, making measurement of maternal iodine status at birth an important marker for the previous fetal environment.[39,42,73–75]
To address the potential postnatal iodine deficiency in neonates, in one report very low birth-weight neonates (<33 weeks postconceptual age; <1500g birth weight) were fed formula supplemented with iodine to achieve a dosage of either ≈10 or 40 μg/kg/day.[96] The authors found no differences in blood levels of T4, T3, or free T4 in relation to iodine intake. However, iodine treatment was not initiated until the neonates were older than 2 postnatal weeks of age and were receiving full oral feeds, and unfortunately, early exposure to iodine was not quantified; each of these confounders may have obviated any later effects. Thus, to date, the relative contribution of these differing influences on iodine balance in ELBW neonates has not been fully assessed from birth.
4. Closing Thoughts and Perspectives
The only study that has investigated THOP and its association with disabling cerebral palsy found a 4-fold increased risk of cerebral palsy in the 15% of infants of <33 weeks’ gestational age who had the lowest T4 levels.[3] This relative risk of four, as associated with cerebral palsy, translates into an etiologic traction of 75%, and a population attributable risk of 31%. This means that 75% of all disabling cerebral palsy, in children who experience THOP, and nearly one-third of disabling cerebral palsy in all infants born at <33 weeks’ gestation age, is associated with low thyroid hormone levels. Approximately 20 000 births each year in the US occur at <28 weeks’ gestation and 70% of these neonates (≈14 000) survive. Approximately 12% of survivors, nearly 1700 children, will have disabling cerebral palsy.[2,80] In addition, several studies have measured the IQ or its equivalent in such children and found a reduction of seven to eight points (or more than halt a standard deviation tor the population) in the children of mothers who experienced subclinical hypothyroidism during pregnancy, independent of THOP, suggesting the problem of thyroid hormone insufficiency may be even more widespread (table I).[39,73,74,76,77] Thus, modeling could not explain all of the variability in the occurrence of cerebral palsy in neonates born at ≤28 weeks of gestational age, although, in theory, treatment of THOP alone could lead to the prevention of as many as 500–600 cases of cerebral palsy per year in this gestational age group.
Paneth[4] reviewed relationships among THOP, adverse neurologic outcomes, and other perinatal variables, and described six different ways in which these sets of variables could be related to each other, only some of which implied a causal role tor THOP in the neurologic outcomes. However, unlike many other risk factors discovered in population-based clinical research, the association of THOP with adverse neurologic outcomes is supported by a solid body of laboratory and clinical evidence, including the well known adverse effects of thyroid and iodine deticiency on the brain (table I).[39,73,76,77,97]
From population surveys and perinatal, developmental, human, animal, and cell culture data, there is clearly a CNS window of vulnerability tor brain damage in ELBW neonates. What is not yet known, and what cannot be established by any means other than a properly powered interventional trial, is whether the strong association of THOP with impaired neurodevelopment is in tact causal. Since previous work could not prove the need to treat due to small sample sizes and the concern that excessive treatment is itself a risk, outright intervention is not advocated at this time. Unfortunately, the lack of a mandate to intercede is not the same as feeling reassured in taking no action. Indeed, a recent survey indicated contusion in the field since some clinicians treat, others never treat, and many do not know what to do.[98] Therefore, we suggest that the individual clinician must review the balance of evidence and formulate their approach as to whether or not they choose to treat and it so, they must set a priori goals with regard to the dose, duration, and mode of thyroid hormone therapy to achieve a targeted blood level. For example, only supplementing to levels equivalent to those provided by endogenous production and in quantities low enough to avoid suppression of TSH release would be comparable to goals set tor thyroid hormone treatment in older age groups.[85] Whatever path is chosen, staying on it will enable future reflection on results rather than risking random intervention based on physician-to-physician bias in a constantly changing world of perinatal interventions.[98]
References
1. PeriStats. March of Dimes Perinatal Data Center [online]. Available from URL: http://www.marchofdimes.com/peristats [Accessed 2006 Oct 16]
Lorenz JL, Wooliever DE, Jetton J, et al. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med 1998; 152: 425–535
Reuss ML, Paneth N, Pinto JA, et al. The relation of transient hypothyroxinemia in preterm infants to neurological development at 2 years of age. N Engl J Med 1996; 334: 821–7
Paneth N. Does transient hypothyroxinemia cause abnormal neurodevelopment in premature infants? Clin Perinatol 1998; 25: 627–37
Honeycutt A, Dunlap L, Chen H, et al. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment: United States, 2003. MMWR Morb Mort Weekly Rep 2003; 53: 57
van Wassenaer AG, Kok JH, Dekker FW, et al. Thyroid function in very preterm infants: influence of gestation age and disease. Pediatr Res 1997; 42: 604–9
Simpson J, Williams FLR, Delahunty C, et al. Serum thyroid hormones in preterm infants and relationships to indices of severity of intercurrent illness. J Clin Endocrinol Metab 2005; 90: 1271–9
Williams FLR, Ogston SA, van Toor H, et al. Serum thyroid hormones in preterm infants: associations with postnatal illnesses and drug usage. J Clin Endocrinol Metab 2005; 90: 5954–63
Meijer WJ, Verloove-Vanhorick SP, Brand R, et al. Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Arch Dis Child 1992; 67: 944–7
DenOuden AL, Kok JH, Verkerk PH, et al. The relation between neonatal thyroxine levels and neurodevelopmental outcome at 5 and 9 years in a national cohort of preterm and low birth weight infants. Pediatr Res 1996; 39: 142–5
Lucas A, Morley R, Fewtrell MS. Low triiodothyronine concentration in preterm infants and subsequent intelligence quotient (IQ) at 8 year follow-up. BMJ 1996; 312: 1132–3
Paul DA, Leef KH, Stefano JL, et al. Low serum thyroxine on initial newborn screening is associated with intraventricular haemorrhage and death in very low birth weight infants. Pediatrics 1998; 101: 903–7
Leviton A, Paneth N. Hypothyroxinemia of prematurity and the risk of cerebral white matter changes. J Pediatr 1999; 134: 706–11
Pop VJ, Brouwers EP, Vader HL, et al. Maternal hypothyroxinemia during early pregnancy and subsequent child development: 3 year follow-up. Clin Endocrinol (Oxf) 2003; 59: 282–8
Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol 2004; 151Suppl. 3: U25–37
Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol 2004; 16: 809–18
De Groot LJ. Dangerous dogmas in medicine: the non-thyroidal illness syndrome. J Clin Endocrinol Metab 1999; 84(1): 151–64
Fisher DA. Thyroid function in premature infants: the hypothyroxinemia of prematurity. Clin Perinatol 1998; 25: 999–1014
Rapaport R, Rose SR, Freemark M. Hypothroxinemia in the preterm infant: the benefits and risks of thyroxine treatment. J Pediatr 2001; 139: 182–8
Rabin CW, Hopper AO, Job L, et al. Incidence of low free T4 values in premature infants as determined by equilibrium dialysis. J Perinatol 2004; 24: 640–4
Boelaert K, Franklyn JA. Thyroid hormone in health and disease. J Endocrinol 2005; 187: 1–15
Chopra IJ. Euthyroid sick syndrome: is it a misnomer? J Clin Endocrinol Metab 1997; 82: 329–34
Tilotson SL, Fuggle PW, Smith I, et al. Relation between biochemical severity and intelligence in early treated congenital hypothyroidism: a threshold effect. BMJ 1994; 309: 440–5
Vanhole C, Aerssens P, Naulaers G. L-thyroxine treatment of preterm newborns: clinical and endocrine effects. Pediatr Res 1997; 42: 87–92
Chowdry P, Scanlon JW, Auerbach R, et al. Results of a controlled double-blind study of thyroid replacement in very-low-birth-weight premature infants with hypothyroxinemia. Pediatrics 1984; 73: 301–5
Schonberger W, Grimm W, Emmrich P, et al. Reduction in mortality rate in premature infants by substitution of thyroid hormones. Eur J Pediatr 1981; 135: 245–53
Amato M, Pasquier S, Carasso A, et al. Postnatal thyroxine administration for idiopathic respiratory distress syndrome in premature infants. Horm Res 1988; 29: 27–30
Amato M, Guggisberg C, Schneider H. Postnatal triiodothyronine replacement and respiratory distress syndrome of the preterm infant. Horm Res 1989; 32: 213–7
van Wassenaer AG, Kok JH, de Vijlder JJM, et al. Effects of thyroxine supplementation on neurological development in infants born at less than 30 weeks gestation. N Engl J Med 1997; 336: 21–6
Osborne DA. Thyroid hormone for preventing neurodevelopment impairment in preterm infants [Cochrane Review]. In: The Cochrane Library, issue 4: dy2001 [online]. Available from URL: http://www.thecochranelibrary.com [Accessed 2006 Jun 29]
Smith LM, Leake RD, Berman N, et al. Postnatal thyroxine supplementation in infants less than 32 weeks: effects on pulmonary morbidity. J Perinatol 2000; 20: 427–31
Valerio PG, van Wassenaer AG, de Vijlder JJ, et al. A randomized, masked study of triiodothyronine plus thyroxine administration in preterm infants less than 28 weeks of gestational age: hormonal and clinical effects. Pediatr Res 2004; 55:248–53
Williams FLR, Mires GJ, Barnett C, et al. Transient hypothyroxinemia in preterm infants: the role of cord sera thyroid hormone levels adjusted for prenatal and intrapartum factors. J Clin Endocrinol Metab 2005; 90: 4599–606
Briet JM, van Wassenaer AG, Dekker FW, et al. Neonatal thyroxine supplementation in very preterm children: developmental outcome evaluated at early school age. Pediatrics 2001; 107(4): 712–8
Briet JM, van Wassenaer AG, van Baar A, et al. Evaluation of the effect of thyroxine supplementation on behavioral outcome in very preterm infants. Develop Med Child Neurol 1999; 41: 87–93
van Wassenaer AG, Briët JM, van Baar A, et al. Free thyroxine levels during the first weeks of life and neurodevelopmental outcome until the age of 5 years in very preterm infants. Pediatrics 2002; 110: 534–9
Briet JM, van Sonderen L, Buimer M, et al. Neurodevelopmental outcome of children treated with antenatal TRH. Pediatrics 2002; 110: 249–53
Ballard PL, Ballard RA, Creasy RK, et al. Plasma thyroid hormones and prolactin in premature infants and their mothers after prenatal treatment with thyrotropin-releasing hormone. Pediatr Res 1992; 32: 673–8
Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev 1997; 18: 404–33
Thorpe-Beeston JG, Nicolaides KH, Felton CV, et al. Maturation of the secretion of thyroid hormone and thyroid stimulating hormone in the fetus. New Engl J Med 1991; 324: 532–6
Fisher DA. Development of fetal thyroid system control. In: Delong GR, Robbins J, Condliffe PG, editors. Iodine and the brain. New York: Plenum Publishing Corp., 1989: 167–76
Ares S, Escobar-Morreale HF, Quero J, et al. Neonatal hypothyroxinemia: effects of iodine intake and premature birth. J Clin Endocrinol Metab 1997; 82: 1704–12
Murphy N, Hume R, van Toor H, et al. The hypothalamic-pituitary-thyroid axis in preterm infants; changes in the first 24 hours of postnatal life. J Clin Endocrinol Metab 2004; 89: 2824–31
Morreale de Escobar G, Ares S. The hypothyroxinemia of prematurity. J Clin Endocrinol Metab 1998; 83: 713–5
Richard K, Hume R, Kaptein E, et al. Ontogeny of iodothyronine deiodinases in human liver. J Clin Endocrinol Metab 1998; 83: 2868–74
Kahaly GJ, Dillman WH. Thyroid hormone action in the heart. Endocrine Rev 2005; 26: 704–28
Rajatapiti P, Kester MHA, de Krijger RR, et al. Expression of glucocorticoids, retinoid, and thyroid hormone receptors during human lung development. J Clin Endo Metab 2005; 90: 4309–14
van Wassenaer AG, Kok JH, Briet JM, et al. Thyroid function in very preterm newborns: possible implications. Thyroid 1999; 9: 85–91
Mackie AS, Booth KL, Newburger JW, et al. A randomized, double-blind, placebo-controlled pilot trial of T3 in neonatal heart surgery. J Thoracic Cardiovasc Surg 2005; 130: 810–6
Moog F. The differentiation and redifferentiation of the intestinal epithelium and its brush border membrane. Ciba Foundation Symp 1979; 70: 31–50
Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 1994; 6: 141–50
Hume R, Richard K, Kaptein E, et al. Thyroid hormone metabolism and the developing human lung. Biol Neonate 2001; 80Suppl. 1: 18–21
Ramadurai SM, Nielsen HC, Chen Y, et al. Differential effects in vivo of thyroid hormone on the expression of surfactant phospholipid, surfactant protein mRNA and antioxidant enzyme mRNA in fetal rat lung. Exp Lung Res 1998; 24: 641–57
Reyes G, Romaguera J, Zapata R, et al. Effect of prenatal T4 treatment in neonatal morbidity: preliminary findings. Puerto Rico Health Sci J 1997; 16(1): 5–8
Biswas S, Buffery J, Enoch H, et al. Pulmonary effects of triiodothyronine (T3) and hydrocortisone (HC) supplementation in preterm infants less than 30 weeks gestation: results of the THORN trial. Thyroid Hormone Replacement in Neonates. Pediatr Res 2003; 53: 48–56
Aden D, La Gamma EF, Browne LE. Nutritional management and the multisystem organ failure/systemic inflammatory response syndrome in critically ill preterm neonates. Crit Care Clin 1995; 11(3): 751–70
Cageao LF, Mignone IR, Ricci CR, et al. Effects of thyroid hormones on mitochondrial oxygen consumption in brown adipose tissue and heart from cold-exposed hypothyroid rats. Acta Endocrinol (Copenh) 1992; 127: 72–5
Polk DH. Thyroid hormone effects on neonatal thermogenesis. Semin Perinatol 1988; 12: 151–6
Peeters RP, Wouters PJ, Kaptein E, et al. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab 2003; 88: 3202–11
Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med 1999; 130: 750–8
Robson H, Siebler T, Shalet SM, et al. Interactions between GH, IGF-1, glucocorticoids and thyroid hormones during skeletal growth. Pediatr Res 2002; 52(2): 137–47
Bianco AC, Silva JE. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 1987; 79: 295–300
Gluckman PD, Sizonenko SV, Bassett NS. The transition from fetus to neonate: an endocrine perspective. Acta Paediatr Suppl 1999; 428: 7–11
Fisher DA, Klein AH. Thyroid development and disorders of thyroid function in the newborn. N Engl J Med 1981; 304: 702–12
Ng PC, Lee CH, Lam CW, et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2004; 89: F119–26
Yeung M, Smyth JP. Hormonal factors in the morbidities associated with extreme prematurity and the potential benefits of hormonal supplement. Biol Neonate 2002; 81(1): 1–15
Bruhn TO, Bolduc TG, Rondeel JMM, et al. Thyrotropin-releasing hormone gene expression in the anterior pituitary: II. Stimulation by glucocorticoids. Endocrinology 1994; 134: 821–5
Van der Geyten S, Darras VM. Developmentally defined regulation of thyroid hormone metabolism by glucocorticoids in the rat. J Endocrinol 2005; 185: 327–36
Surks MI, Sievert R. Drugs and thyroid function. New Engl J Med 1995; 333: 1688–94
Williams FLR, Simpson J, Delahunty C, et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J Clin Endocrinol Metab 2004; 89: 5314–20
Huang W, Wood C, L’ Abbe MR, et al. Soy protein isolate increases hepatic thyroid hormone receptor content and inhibits its binding to target genes in rats. J Nutr 2005; 135: 1631–5
Conrad SC, Chiu H, Silverman BL. Soy formula complicates management of congenital hypothyroidism. Arch Dis Child 2004; 89: 37–40
Cao XY, Jiang XM, Dou ZH, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med 1994; 331: 1739–44
Hollowell JG, Staehling NW, Hannon WH, et al. Iodine nutrition in the United States: trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab 1998; 83: 3401–8
Dunn JT. Editorial: what’s happening to our iodine? J Clin Endocrinol Metab 1998; 83: 3398–400
Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in T4 requirements during pregnancy in women with hypothyroidism. N Engl J Med 2004; 351: 241–9
Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341: 549–55
Ares S, Quero J, Morreale de Escobar G. Neonatal iodine deficiency: clinical aspects. J Pediatr Endocrinol Metab 2005; 18: 1257–64
Ogilvy-Stuart AL. Neonatal thyroid disorders. Arch Dis Child 2002; 87: F165–71
Ares S, Quero J, Durán S, et al. Iodine content of infant formulas and iodine intake of premature babies: high risk of iodine deficiency. Arch Dis Child 1994; 71: 184–91
Ares S, Pastor I, Quero J, et al. Thyroid complications, including overt hypothyroidism, related to the use of non-radiopaque silastic catheters for parenteral feeding in prematures requiring injection of small amounts of an iodinated contrast medium. Acta Pediatr 1995; 84: 579–81
Wolff J, Chaikoff IL. The Wolff-Charkoff effect. J Biol Chem 1948; 172: 855–6
O’Brien CA, Blumer JL, Speck WT, et al. Effect of bathing with a 4% chlorhexidine gluconate solution on neonatal bacterial colonization [letter]. J Hosp Infect 1984; 5: 141
Rovet J. Congenital hypothyroidism: treatment and outcome. Curr Opin Endocrinol Diabetes 2005; 12: 42–52
van Trotsenburg ASP, Vulsma T, Rutgers van Rozenburg-Marres SL, et al. The effect of thyroxine treatment started in the neonatal period on development and growth of two year old Down Syndrome children: a randomized clinical trial. J Clin Endocrinol Metab 2005; 90: 3304–11
Pilo A, Iervasi G, Vitek F, et al. Thyroid and peripheral production of 3,5,3′-triiodothyronine in humans by multi-compartmental analysis. Am J Physiol 1990; 258: E715–26
Kester MH, Martinez de Mena R, Obregon MJ, et al. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab 2004; 89: 3117–28
Calvo RM, Obregón MJ, Ruiz de Oña C, et al. Congenital hypothyroidism, as studied in rats: crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest 1990; 86: 889–99
van Wassenaer AG, Kok JH, Endert E, et al. Thyroxine supplementation to infants of less than 30 weeks gestational age does not increase plasma triiodothyronine concentrations. Acta Endocrinol (Copenh) 1993; 129: 139–46
van Wassenaer AG, Kok JH, Endert E, et al. Thyroxine supplementation to infants of less than 30 weeks gestational age decreases plasma triiodothyronine concentrations. Eur J Endocrinol 1998; 139: 508–15
Escobar-Morreale HF, Escobar del Rey FE, Obregon MJ, et al. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 1996; 137: 2490–502
Cools F, Van Wassenaer AG, Kok JH, et al. Changes in plasma thyroid hormone levels after a single dose of triiodothyronine in premature infants of less than 30 weeks gestational age. Eur J Endocrinol 2000; 143: 733–7
Hume R, Simpson J, Delahunty C, et al. Human fetal and cord serum thyroid hormones: developmental trends and interrelationships. J Clin Endocrinol Metab 2004; 89: 4097–103
Vulsma T, Gons MH, de Vijlder JJM. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 1989; 321: 13–6
Delange F, Dunn, JT, Glinoer D. Recommendations on iodine nutrition for mothers and infants in Europe. In: Delange F, Dunn JT, Glinoer D, editors. Iodine deficiency disorders in Europe: a continuing concern. New York: Plenum Press, 1993: 471–8
Rogahn J, Ryan S, Wells J, et al. Randomized trial of iodine intake and thyroid status in preterm infants. Arch Dis Child Fetal Neonatal Ed 2000; 83: F86–90
Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab 2004; 89(12): 6054–60
Golombek GG, La Gamma EF, Paneth N. Treatment of transient hypothyroxinemia of prematurity: a survey of neonatal practice. J Perinatol 2002; 22: 563–4
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
La Gamma, E.F., van Wassenaer, A.G., Golombek, S.G. et al. Neonatal Thyroxine Supplementation for Transient Hypothyroxinemia of Prematurity. Mol Diag Ther 5, 335–346 (2006). https://doi.org/10.2165/00024677-200605060-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200605060-00002