Abstract
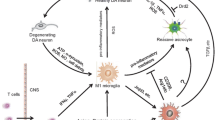
There is ample and increasing evidence, from studies of human pathology, animal models and tissue culture, that chronic inflammation occurs in the basal ganglia in patients with Parkinson’s disease. In such inflammatory states, activated glia can produce large quantities of free radicals and other neurotoxic materials. Dopaminergic neurons appear to be particularly vulnerable to these neurotoxins.
The anti-inflammatory drugs that are presently in wide use act on peripheral players in the inflammatory process. Many experiments are under way to find agents that inhibit more potent contributors, such as the activated microglia or terminal complement proteins. Whether such drugs will slow the process of Parkinson’s disease or reduce the high risk of dementia in such patients remains to be determined in future work.



Similar content being viewed by others
References
Douglas MR, Lewthwaite AJ, Nicholl DJ. Genetics of Parkinson’s disease and parkinsonism. Expert Rev Neurother 2007; 7(6): 657–66
Casarejos MJ, Menendez J, Solano RM, et al. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. J Neurochem 2006; 97: 934–46
Hattori N, Machida Y, Sato S, et al. Molecular mechanisms of nigral neurodegeneration in Park2 and regulation of parkin protein by other proteins. J Neural Transmiss Suppl 2006; 70: 205–8
Sun M, Latourelle JC, Wooten GF, et al. Influence of heterozygosity for parkin mutation on onset age in familial Parkinson disease: the GenePD study. Arch Neurol 2006; 63: 826–32
Smith WW, Pei Z, Jiang H, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A 2005; 102: 18676–81
West AB, Moore DJ, Biskup S, et al. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A 2005; 102: 16842–7
Kachergus J, Mata IF, Hulihan M, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet 2005; 76: 672–80
Whaley NR, Uitti RJ, Dickson DW, et al. Clinical and pathologic features of families with LRRK2-associated Parkinson’s disease. J Neural Transmiss Suppl 2006; 70: 221–9
Ishihara L, Warren L, Gibson R, et al. Clinical features of Parkinson disease patients with homozygous leucine-rich repeat kinase 2 G2023S mutations. Arch Neurol 2006; 63: 1250–4
Kay DM, Zabetian CP, Factor SA, et al. Parkinson’s disease and RRK2: frequency of a common mutation in US movement disorder clinics. Movement Dis 2006; 21: 519–23
Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004; 44: 601–7
Davidzon G, Greene P, Mancuso M, et al. Early-onset familial parkinsonism due to POLG mutations. Ann Neurol 2006; 59: 859–62
Skovronsky DM, Lee VMY, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol Mech Dis 2006; 1: 151–70
Miklossy J, Arai T, Guo J-P, et al. LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropath Exp Neurol 2006; 65: 953–63
Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314: 130–3
McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging 2007; 28(5): 639–47
McGeer PL, Sibley J. Sparing of age-related macular degeneration in rheumatoid arthritis. Neurobiol Aging 2006; 37: 1533–4
Kurkowska-Jastrzebska I, Wroska A, Kohutnicka M, et al. MHC class II positive microglia and lymphocytic infiltration are present in the substantia nigra and striatum in mouse model of Parkinson’s disease. Acta Neurobiol Exp 1999; 59: 1–8
Kurkowska-Jastrzebska I, Wroska A, Kohutnicka M, et al. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol 1999; 156: 50–61
McGeer PL, Itagaki S, Akiyama H, et al. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol 1988; 24: 574–6
Miklossy J, Doudet DD, Schwab C, et al. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol 2006; 197: 275–83
Le WD, Rowe DB, Xie W. Activated microglia induce dopaminergic cell injury in vitro. XIII International Congress on Parkinson’s Disease, Parkinsonism, and Related Disorders; 1999 Jul 24–28; Vancouver (BC). 5(2): S19
Gao HM, Hong JS, Zhang W, et al. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. J Neurosci 2003; 23: 1228–36
Chung ES, Joe EH, Ryu JK, et al. GT1b ganglioside induces death of dopaminergic neurons in rat mesencephalic cultures. Neuroreport 2001; 12: 611–4
Gao HM, Hong JS, Zhang W, et al. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 2002; 22: 782–90
He Y, Le WD, Appel SH. Role of Fcgamma receptors in nigral cell injury induced by Parkinson disease immunoglobulin injection into mouse substantia nigra. Exp Neurol 2002; 176: 322–7
Yamada T, McGeer PL, McGeer EG. Relationship of complement-activated oligodendrocytes to reactive microglia and neuronal pathology in neurodegenerative disease. Dementia 1991; 2: 71–7
McGeer PL, Itagaki S, Boyes BE, et al. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988; 38: 1285–91
Orr CF, Rowe DB, Mizuno Y, et al. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain 2005; 128: 2665–74
Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transmiss Suppl 2006; 70: 373–81
Yamada T, McGeer EG, Schelper RL, et al. Histological and biochemical pathology in a family with autosomal dominant Parkinsonism and dementia. Neurol Psychiatry Brain Res 1993; 2: 26–35
Langsten JW, Forno LS, Tetrud J, et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 1999; 46: 598–605
McGeer PL, Schwab C, Parent A, et al. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine administration. Ann Neurol 2003; 54: 599–604
McGeer PL, Yasojima K, McGeer EG. Inflammation in Parkinson’s disease. Adv Neurol 2001; 86: 83–9
Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol 2003; 53Suppl. 3: S26–36
Koutsilieri E, Scheller C, Grunblatt E, et al. Free radicals in Parkinson’s disease. J Neurol 2002; 249Suppl. 2: II1–5
Gerhard A, Pavese N, Hotten G, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 2006; 21: 404–12
Ouchi Y, Yoshikawa E, Sekine Y, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol 2005; 57: 168–75
Cicchetti F, Brownell AL, Williams K, et al. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci 2002; 15: 991–8
Sugama S, Yang L, Cho BP, et al. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/ 6 mice. Brain Res 2003; 964: 288–94
Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 2003; 23: 6181–7
Sherer TB, Betarbet R, Kim JH, et al. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci Lett 2003; 341: 87–90
Douhou A, Debeir T, Michel PP, et al. Differential activation of astrocytes and microglia during post-natal development of dopaminergic neuronal death in the weaver mouse. Dev Brain Res 2003; 145: 9–17
Arimoto T, Bing G. Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol Dis 2003; 12: 35–45
Iravani MM, Kashefi K, Mander P, et al. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience 2002; 110: 49–58
Ryu JK, Shin WH, Kim J, et al. Trisialoganglioside GT1b induces in vivo degeneration of nigral dopaminergic neurons: role of microglia. Glia 2002; 38: 15–23
McLaughlin P, Zhou Y, Ma T, et al. Proteomic analysis of microglial contribution to mouse strain-dependent dopaminergic neurotoxicity. Glia 2006; 53: 567–82
Shavali S, Combs CK, Ebadi M. Reactive macrophages increase oxidative stress and alpha-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson’s disease. Neurochem Res 2006; 31: 85–94
Walker DG, Lue L-F, Klegeris A, et al. The involvement of glial cell-derived reactive oxygen and nitrogen species in Alzheimer’s disease. In: Rogers J, editor. Neuroinflammatory mechanisms in Alzheimer’s disease: basic and clinical research. Basel: Birkhauser Verlag, 2001: 173–95
Herrera AJ, Castano A, Venero JL, et al. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on the dopaminergic system. Neurobiol Dis 2000; 7: 429–47
Gao HM, Jiang J, Wilson B, et al. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem 2002; 81: 1285–97
Kim WG, Mohney RP, Wilson B, et al. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci 2000; 20: 6309–16
Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 2005; 19: 533–42
Ciesielski-Treska J, Ulrich G, Taupenot L, et al. Chromogranin A induces a neurotoxic phenotype in brain microglial cells. J Biol Chem 1998; 273: 14339–46
Yasuhara O, Kawamata T, Aimi Y, et al. Expression of chromogranin A in lesions of the central nervous system from patients with neurological diseases. Neurosci Lett 1994; 170: 13–6
Lee DY, Oh YJ, Jin BK. Thrombin-activated microglia contribute to death of dopaminergic neurons in rat mesencephalic cultures: dual roles of mitogen-activated protein kinase signaling pathways. Glia 2005; 51: 98–110
Ishida Y, Nagai A, Kobayashi S, et al. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J Neuropath Exp Neurol 2006; 65: 66–77
Wilms H, Rosenstiel P, Sievers J, et al. Activation of microglia by human neuromelanin is NF-kappaB dependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J 2003; 17: 500–2
Aimi Y, McGeer PL. Lack of toxicity of human neuromelanin to rat brain dopaminergic neurons. Parkinsonism Relat Disord 1996; 2: 69–74
Mosser DM. The many faces of macrophage activation. J Leuko Biol 2003; 73: 209–12
Mogi M, Harada M, Riederer P, et al. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 1994; 165: 208–10
Boka G, Anglade P, Wallach D, et al. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett 1994; 172: 151–4
Hunot S, Dugas N, Faucheux B, et al. Fc epsilon RII/CD23 is expressed in Parkinson’s disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci 1999; 19: 3440–7
McGeer PL, Yasojima K, McGeer EG. Association of interleukin-1 beta polymorphisms with idiopathic Parkinson’s disease. Neurosci Lett 2002; 326: 67–9
Schulte T, Schols L, Muller T, et al. Polymorphisms in the interleukin-1 alpha and beta genes and the risk for Parkinson’s disease. Neurosci Lett 2002; 326: 70–2
Hakansson A, Westberg L, Nilsson S, et al. Interaction of polymorphisms in the genes encoding interleukin-6 and estrogen receptor beta on the susceptibility to Parkinson’s disease. Am J Med Genetics 2005; 133: 88–92
Hakansson A, Westberg L, Nilsson S, et al. Investigation of genes coding for inflammatory components in Parkinson’s disease. Movement Dis 2005; 20: 569–73
Gayle DA, Ling ZD, Tong CW, et al. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-α, interleukin-1b, and nitric oxide. Dev Brain Res 2002; 133: 27–36
Sriram K, Matheson JM, Benkovic SA, et al. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J 2002; 16: 1474–6
Henze C, Hartmann A, Lescot T, et al. Proliferation of microglial cells induced by 1-methyl-4-phenylpyridinium in mesencephalic cultures results from an astrocyte-dependent mechanism: role of granulocyte macrophage colony-stimulating factor. J Neurochem 2005; 95: 1069–77
Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol 1992; 84: 100–4
Goldknopf IL, Sheta EA, Bryson J, et al. Complement C3c and related protein biomarkers in amyotrophic lateral sclerosis and Parkinson’s disease. Biochem Biophys Res Commun 2006; 342: 1034–9
Chen H, Zhang SM, Herman MA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol 2003; 60: 1059–64
Chen H, Jacobs E, Schwarzschild MA, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson’s disease. Ann Neurol 2005; 58: 963–7
Bower JH, Maraganore DM, Peterson BJ, et al. Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: a case-control study. Neurology 2006; 67: 494–6
Hernan MA, Logroscino G, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology 2006; 66: 1097–9
Peng J, Xie L, Stevenson FF, et al. Nigrostriatal dopaminergic neurodegeneration in the weaver mouse is mediated via neuroinflammation and alleviated by minocycline administration. J Neurosci 2006; 6: 11644–51
Du Y, Ma Z, Lin S, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A 2001; 98: 14669–74
Wu DC, Jackson-Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci 2002; 22: 1763–71
He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res 2001; 909: 187–93
Wang T, Zhang W, Pei Z, et al. Reactive microgliosis participates in MPP+-induced dopaminergic neurodegeneration: role of 67 kDa laminin receptor. FASEB J 2006; 20: 906–15
Li G, Cui G, Tzeng NS, et al. Femtomolar concentrations ofdextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB J 2005; 19: 489–96
Noelker C, Bacher M, Gocke P, et al. The flavanoide caffeic acid phenethyl ester blocks 6-hydroxydopamine-induced neurotoxicity. Neurosci Lett 2005; 383: 39–43
Lastres-Becker I, Molina-Holgado F, Ramos JA, et al. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson’s disease. Neurobiol Dis 2005; 19: 96–107
Fahrig T, Gerlach I, Horvath E. A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson’s disease and traumatic brain injury. Mol Pharmacol 2005; 67: 1544–55
Zhang W, Qin L, Wang T, et al. 3-Hydroxymorphinan is neurotrophic to dopaminergic neurons and is also neuroprotective against LPS-induced neurotoxicity. FASEB J 2005; 19: 395–7
McCoy MK, Martinez TN, Ruhn KA, et al. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci 2006; 26: 9365–75
Zoccarato F, Toscano P, Alexandre A. Dopamine-derived dopaminochrome promotes H(2)O(2) release at mitochondrial complex I: stimulation by rotenone, control by Ca(2+), and relevance to Parkinson disease. J Biol Chem 2005; 280: 15587–94
Choi DK, Pennathur S, Perier C, et al. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci 2005; 25: 6594–600
Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nature Neurosci 2006; 9: 917–24
Acknowledgements
The preparation of this review was supported by grants from the Pacific Parkinson’s Research Institute, the Parkinson Foundation of Canada, and the Jack Brown and Family A.D. Research Fund, as well as donations from the estate of George Hodgson, The Friends of UBC and individual persons from the province of British Columbia. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGeer, E.G., McGeer, P.L. The Role of Anti-Inflammatory Agents in Parkinson’s Disease. CNS Drugs 21, 789–797 (2007). https://doi.org/10.2165/00023210-200721100-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200721100-00001