Abstract
Indications for the use of antimicrobials in critically ill patients are similar to those for other hospitalised patients. However, the selection of agents depends on the particular characteristics of patients in the intensive care unit (ICU), the form of presentation of infection, the type of infection and the bacteriological features of the causative pathogens.
The use of antimicrobials in patients admitted to medical-surgical ICUs varies between 33 and 53%. The selection of empirical antimicrobials to be included in treatment protocols of the most common infections depends on the strong interrelationship between patient characteristics, predominant pathogens in each focus, and antimicrobials used for treatment.
Epidemiological studies carried out in the past have identified the microorganisms most frequently responsible for community-acquired and nosocomial infections in patients admitted to ICUs. Susceptibility to antimicrobial agents may be different between each geographical area, between each hospital and even within the same hospital service. In addition, susceptibility patterns may change temporarily in relation to the use of particular antimicrobials or in association with other unknown factors so that assessment of endemic antimicrobial resistance patterns is very useful in order to tailor the antimicrobial regimens of therapeutic protocols.
Antimicrobial use should not be a routine procedure. The clinical course of the patient (an indicator of effectiveness) should be closely monitored as well as the possible appearance of adverse effects and/or multiresistant pathogens. Controls are based on the assessment of plasma drug concentrations and microbiological surveillance to detect the presence of multiresistant strains or new antibacterial-resistant pathogens.
Prevention of the development of multiresistant pathogens is the main goal of the ICU antimicrobial policy. Although a series of general strategies to reduce the presence of multiresistant pathogens have been proposed, the implementation of these recommendations in ICUs requires the cooperation of a member of the intensive care team.
Similar content being viewed by others
Over half of all patients admitted to intensive care units (ICUs) receive one or more antimicrobial agent, and most of these are for the treatment of community-acquired and/or nosocomial infections.[1–3] Indications for the use of antimicrobials are similar to those for other hospitalised patients, but the selection of agents depends on the particular characteristics of patients in the ICU (severity of illness, immunosuppression, multiorgan failure, high distribution volume), the form of presentation of infections (severe sepsis, septic shock, coagulation disorders, respiratory failure), the type of infection (catheter-related bacteraemia, mechanical ventilation-associated pneumonia), and the bacteriological features of causative pathogens. Numerous studies have confirmed the relationship between antibacterial usage and the development of resistance among both community and hospital bacterial pathogens, as well as for an increased risk of colonisation by hospital pathogens.[4–9] These phenomena are especially relevant for ICUs, in which the development of nosocomial infection is frequent for different reasons and the presence of multiresistant pathogens may be a determining factor for the outcome of patients.
In most patients, the use of antimicrobials is started before identification of the causative pathogen (empirical treatment). The selection of antimicrobial agents for protocols of empirical treatment of infections in ICU patients is as a result of careful consideration of the problem taking into account 3 essential elements: pathogens and their susceptibility patterns, antimicrobials and patients.
Between 25 and 50% of antimicrobial prescriptions may be incorrect for reasons of indication, choice, dosage or duration of therapy.[10,11] Recognition of the consequences of abuse and misuse of antimicrobials prompted the development of a series of guidelines and recommendations to improve antimicrobial usage. Implementation of these recommendations in the ICU setting involves additional difficulties than for other in-patient settings.
The aim of this review is to describe factors of critical importance in the use of antimicrobials in patients hospitalised in the ICU, to discuss how the use of antimicrobials in this patient population can be best managed in the current healthcare environment, and to summarise recommendations based on experts’ opinions to improve antimicrobial prescribing patterns.
1. Use of Antimicrobials in the Intensive Care Unit (ICU)
Different multicentre studies carried out in the past have provided data on antibacterial use in ICUs. The European Prevalence of Infection in Intensive Care (EPIC study) showed that 62.3% of patients admitted to the participating ICUs had received one or more antibacterial agent on the day of data collection.[1] A study of the prevalence of nosocomial infection in Spain (EPINE study) revealed that 51% of critically ill patients (including transplant patients) were given antibacterials, which accounted for 5% of the overall antibacterial consumption in the hospital.[2] According to a national study on surveillance of nosocomial infection in ICU (ENVIN-ICU study) carried out in Spain since 1994 by the Sociedad Española de Medicina Intensiva, Critica y Unidades Coronarias (SEMICYUC) working party of infectious diseases, the use of antibacterials in patients admitted to medical-surgical ICUs varied between 33 and 53% (table I).[3] The severity of the condition of patients admitted to ICUs and their prolonged hospital stay usually determine the prescription of more than one antimicrobial regimen with combinations of different antimicrobials.[3] The most commonly used agents include broad spectrum antibacterials, particularly third generation cephalosporins, aminoglycosides, broad spectrum penicillins and carbapenems, as well as those antibacterials prescribed for treating multiresistant Gram-positive infections, such as glycopeptides (table II).
Antibacterial use in patients admitted to intensive care units (ICUs) [ENVIN-ICU Study, 1994–1998][3]
Changes in antibacterial use in patients admitted to intensive care units (ICUs) [ENVIN-ICU Study, 1994–1998][3]
2. Characteristics of the Use of Antimicrobials in Critically Ill Patients
In critically ill patients, antimicrobials are prescribed early in the presence of clinical suspicion of an infection process that may have an effect on the patient’s final outcome (bacteraemias, pneumonia, meningitis, etc.). In critically ill patients with abdominal infection and ventilator-associated pneumonia, early administration of adequate antimicrobial cover has been shown to be an independent predictor of outcome.[12–14]
Infection is suspected on clinical grounds according to detection of clinical signs of a systemic inflammatory response (fever, tachycardia, tachypnoea, chills). These nonspecific signs associated with other signs of focality (crepitation, purulent bronchial secretion, stiffness in the neck, coma, exudate or pus in the catheter insertion site, etc.) allow the selection of the most adequate antimicrobials for each case according to specific therapeutic protocols for each type of infection, which should be adapted to the characteristics of each hospital.
Other patients are given antimicrobial prophylaxis (intravenous, topical) during the postoperative course of different surgical procedures or to prevent infections in extremely severe conditions (transplantation, immunosuppression, polytrauma).
The use of antimicrobials in critically ill patients should follow a series of rules that are common to antimicrobial usage in other hospitalised patients.
2.1 Collection of Significant Samples of the Infection Foci Before Antimicrobial Administration
Starting antimicrobial therapy should be preceded, in all patients, by collection of safety samples (blood, body fluids, exudates, low pulmonary secretions, or other samples that are considered to be appropriate for each focus) using invasive procedures if needed (fibreoptic bronchoscopy, fluoroscopic-guided needle aspiration, etc.). Occasionally, isolation of pathogens in some samples, such as bronchial secretions, urine or drainage fluid, do not confirm the presence of infection (it may only indicate colonisation) and, in these situations, the relationship of these findings with the patient’s clinical manifestations should be evaluated. In other situations, isolation of pathogens allows the aetiology of infection to be established and, in some clinical situations (e.g. bacteraemia, pneumonia, meningitis), the confirmation of the presence of infection (i.e. a definitive diagnosis).
2.2 Empirical Regimens Using Combinations of Broad Spectrum Antibacterials.
Empirical treatment with broad spectrum bactericidal agents, and frequently with combinations of these agents to increase the therapeutic range or the bactericidal action, is based on initial severity of the patient’s condition, additional infection-attributed mortality, time delay in the availability of results of bacteriological studies, and the low sensitivity and/or specificity of some diagnostic methods. Antibacterials should be administered by the intravenous route to quickly obtain a high plasma and tissue drug concentrations. Some authors believe that early use of adequate empirical antibacterials is the most important factor for a favourable evolution in patients with pneumonitis or peritonitis.
2.3 Use of Maximal Doses
The first dose of antimicrobial should be the maximal dose followed by doses adjusted to the patient’s hepatic and renal function. The goal is to attain maximal plasma concentrations (Cmax) several times higher than minimal inhibitory concentration (MIC) of the causative pathogen (Cmax: MIC ratio). Other pharmacodynamic parameters include the area under the concentration time curve (AUC) : MIC ratio and the time interval in which plasma drug concentrations are greater than the MIC.[15] The 2 first parameters, Cmax : MIC ratio and AUC : MIC ratio, are used for antibacterials whose bactericidal activity depends on maximal concentration, such as the aminoglycosides and quinolones,[16,17] whereas the third pharmacodynamic parameter is used for time-dependent killing agents, such as the β-lactams.[18] The use of low or subclinical doses has been related to the selection of multiresistant bacteria.
Table III shows maximal doses of 29 antibacterials and 4 antifungal agents most frequently used in patients in the ICU together with dose adjustments based on creatinine clearance values.
2.4 Directed Treatment
Antimicrobial treatment should be adjusted after identification of the causative pathogen. Antibacterials more active against the isolated pathogens, with better tissue penetration, which are less toxic and with the most reduced bactericidal spectrum should be selected even in patients with favourable clinical evolution. First-choice antimicrobials for treating the most frequently isolated causative pathogens of ICU infections, as well as those to be used in cases of multiresistance, are shown in table IV. A single antimicrobial can be used in the majority of cases (monotherapy), except for pathogens frequently associated with the rapid development of resistance during treatment or with relapses, and/or pathogens associated with poor prognosis, such as infections caused by Pseudomonas aeruginosa.[19–21] The use of 2 or more antimicrobials is recommended in these situations, although no study has demonstrated that combined treatments are more effective.
3. Factors That Influence the Selection of Antimicrobials for Therapeutic Protocols
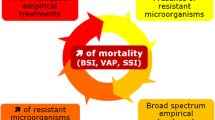
The selection of empirical antimicrobials to be included in treatment protocols of the most common infections depends on the strong inter-relationship between patient characteristics, predominant pathogens in each focus, and antimicrobials used for treatment (fig. 1). Although no study has been designed to specifically address the impact of appropriate empirical therapy on the clinical course of an infection process, different studies have provided evidence for a poor prognosis in patients treated inappropriately.[12–14,22–24]
3.1 Pathogens
Epidemiological studies carried out in the past have identified the micro-organisms most frequently responsible for community-acquired and nosocomial infections in patients in the ICU,[2,11,25–28] as well as the evolution of their antimicrobial susceptibility patterns. These data are useful for selecting those antimicrobial agents which are most appropriate for the treatment of each type of infection. Moreover, it would be useful to have information on the distribution of pathogens in the hospitals through the development of a sequential epidemiological map in which the incidence of the most frequent infections as well as the incidence of the corresponding causative pathogens will be included.
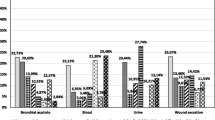
This distribution of nosocomial infections in the ICU varies largely from nosocomial infections in other hospitalised patients, with mechanical ventilation-associated pneumonia, urethral catheter-related urinary tract infection, and catheter-related bacteraemia being the most prevalent. The most frequent causative pathogens for each type of infection in the ENVIN-ICU study[25] are listed in table V.
Aetiological agents for the main nosocomial infections in patients in the intensive care unit (ICU) from the ENVIN-ICU Study, (1994–1998)[25]
Susceptibility to antimicrobial agents may be different between each geographical area, between each hospital and even within the same hospital service. In addition, susceptibility patterns may change temporarily in relation to the use of particular antimicrobials or in association with other unknown factors.[29] This means that assessment of endemic antimicrobial resistance patterns is very useful to tailor the antimicrobial regimens of therapeutic protocols. Personnel responsible for epidemiological surveillance should be alert to the detection of cross-resistance among Gram-negative rods or multiresistance in a particular strain. In these situations, some authors[30,31] have proposed control of the endogenous flora of the patients at risk (bronchial aspirates in mechanically ventilated patients, samples from the oropharynx and rectum in patients with long hospital stay and burn patients, etc.) should be obtained for an early detection of dissemination of the multiresistant strain and to change (if appropriate) the antimicrobials included in empirical therapeutic protocols.
Whenever susceptibility to different antimicrobials is maintained by endemic strains predominating in the hospital service, it would be possible to make an alternative selection, which in turn would prevent the development of new resistance mechanisms by the repeated use of one of them. A progressive increase in the emergence of resistance to a particular agent or to a class of agents should cause temporary discontinuation of its use in both empirical and directed regimens.
3.2 Antimicrobials
Physicians who prescribe antimicrobials should be aware of the main pharmacological characteristics of those agents most commonly administered, including the range of antimicrobial activity, pharmacokinetics, important adverse effects, mechanism of action, clinically relevant interactions, and clinical effectiveness in different indications. The lack of information on some of these aspects may have a negative influence on clinical response to some antimicrobials, such as:
-
limited ability to attain adequate concentrations in the affected tissue (vancomycin in the cerebrospinal fluid, aminoglycosides in bronchial secretions and pancreatic tissue)[32–34]
-
possibility of inactivation by acidic pH (aminoglycosides, imipenem/cilastatin);[35]
-
differences in the post-antibiotic effect (minimal for β-lactams and very high for aminoglycosides).[36]
3.3 Patients and Infections
The characteristics of the patient are the essential factors in determining the causative pathogen. The aetiology of an infection depends on a number of factors, in particular, the immunological status of the patient, which has been associated with some disorders (haematological and oncological diseases, organ transplantation, HIV infection) or some treatments (chemotherapy, corticosteroids). Treatment of opportunistic pathogens frequently found in these patients (fungi, viruses, mycobacteria) should also be considered in protocols of empirical antimicrobial use.
In immunocompetent patients, factors influencing the selection of pathogens are those capable of modifying the primary endogenous flora which predominates in mucous membranes of healthy individuals. Antimicrobial use, prolonged stay in hospital and alteration of natural defensive barriers resulting from insertion of urethral catheters, tracheal tubes or intravascular devices, configurates clearly differentiated risk groups. Accordingly, in the group of patients ‘without risk factors’, causative pathogens are predominantly micro-organisms of the primary endogenous flora present at the time of hospital or ICU admission (Streptococcus pneumoniae, methicillin-susceptible Staphylococcus aureus, Haemophilus influenzae, and Enterobacteriaceae of the digestive tract). These are previously healthy patients admitted to the hospital because of acute conditions (trauma, neurological or surgical) without previous antimicrobial exposure, who develop an infection a few days after hospital admission (primary endogenous infection). Empirical treatment should include the use of antimicrobials that are effective against primary endogenous flora (table VI).
In another group of patients, infections are caused by secondary endogenous flora in which pathogens forming part of the ecosystem of the hospital, with a different distribution in each centre. In the ICUs, this secondary endogenous flora includes P. aeruginosa, Acinetobacter baumannii, and intestinal Enterobacteriaceae (table VI). Other pathogens, such as methicillin-resistant S. aureus (MRSA), extended-spectrum β-lactamase (ESBL) resistance noted predominantly in Klebsiella pneumoniae, or Stenotrophomonas maltophilia that may initially colonise patients at risk and be the cause of new infections, may appear occasionally in a particular area. These are patients admitted to the hospital for many days with different previous antimicrobial regimens with broad spectrum agents and mucosal instrumentation in whom empirical antimicrobials included in therapeutic protocols should be effective against the most prevalent pathogens for a given time at each care area of the critically ill patient.
4. Special Forms of Antimicrobial Administration
4.1 Extended-Interval Aminoglycoside Administration
Single daily doses of aminoglycosides have been recommended in recent years.[37,38] The bactericidal activity of these agents is dose-dependent, with a Cmax : MIC ratio >10 for the optimal dose.[39] This regimen is associated with a Cmax and a greater post-antibiotic effect and has shown a similar efficacy to that of multiple-dose regimens. Two recent meta-analyses[40,41] that included 14 and 21 random studies, respectively, have shown a decrease in nephrotoxicity between 13 and 25% with the use of the extended-interval aminoglycoside dosing (EIAD) regimen. Differences in relation to ototoxicity have not been clearly established. In patients with enterococcal endocarditis, renal insufficiency, and impaired distribution volume, there is insufficient evidence to recommend the use of EIAD.[42] Information on the use of EIAD in pregnant women is not available.
4.2 Continuous Infusion of Antibacterials
Potential advantages of continuous infusion of antibacterials are based on experimental studies with β-lactams especially ceftazidime[43,44] and cefepime.[45] In vitro, β-lactams have shown a time-dependent killing effect. Experience with this modality of antibacterial administration is limited and refers to treatment of P. aeruginosa infection in patients with neutropenia or cystic fibrosis.[46–48] Moreover, there are clinical experiences with the administration of vancomycin[49,50] and flucloxacillin[51] in the treatment of severe infections caused by Gram-positive bacteria.
However, future clinical data will contribute to better define its indication in critically ill patients in whom antibacterial regimens with intermittent doses would have been unsuccessful or in clinical conditions in which concentrations above MIC for most of the administration interval is required.
4.3 Local Antibacterial Administration
The use of multiple combinations of antibacterials may occasionally be unsuccessful in patients with severe infection. In these cases, the presence of multiresistant pathogens makes it necessary to achieve maximal antibacterial concentrations at the site of infection. Although aminoglycosides usually have in vitro activity against these bacteria, their clinical efficacy is limited by poor penetration at the site of infection, especially in bronchial secretions and pulmonary parenchyma. Local administration of antibacterials in the airways to increase antibacterial concentration at the focus of infection has been proposed.[52]
In a double-blind, randomised, placebo-controlled multicentre study, Brown et al.[53] reported no significant differences in the clinical response of bacterial pneumonia, although in the group in which tobramycin was instilled endotracheally, a greater frequency of eradication of the causative pathogens of the pneumonia was achieved. In a noncomparative study, Stoutenbeek et al.[54] achieved excellent results with aerosolised antibacterials (tobramycin plus cefotaxime or ceftazidime) in association with the same antibacterials given intravenously in patients with severe pneumonia who had previously received antibacterials by the gastrointestinal route.
At the present time, evidence of the efficacy of topical administration of antibacterials is limited, but it may be possible that in the future this modality of treatment may be selected in patients with poor prognosis (immunosuppressed, APACHE score >20) or in case of infection caused by high risk pathogens (multiresistant P. aeruginosa and A. baumannii).
5. Controls During Antimicrobial Therapy
Antimicrobial use should not be a routine procedure. The clinical course of the patient (an indicator of efficacy) should be closely monitored in addition to the possible appearance of adverse effects and/or multiresistant pathogens. Controls for antimicrobial use are based on the following criteria.
5.1 Assessment of Plasma Drug Concentrations
Pharmacokinetics of antimicrobial drugs in ICU patients may be altered as a result of haemodynamic changes, renal and/or hepatic failure, generalised oedema, increased volume of distribution, and other complications. Therefore, there is a large between-individual variability in plasma drug concentrations when the same doses are given, so that antimicrobial doses should be optimised, particularly in drugs with a narrow therapeutic range, such as aminoglycosides and vancomycin.[55]
The implementation of pharmacokinetic programmes specifically designed for the therapeutic monitoring of antimicrobial agents, allows dose requirements to be estimated to obtain maximal clinical efficacy with a minimal incidence of adverse effects.[56]
5.2 Microbiological Surveillance to Detect the Presence of Resistant Pathogens
The high level of use of antimicrobial agents in ICUs favours the appearance of multiresistant pathogens, the presence of which is associated with both failure of antimicrobial therapy in a given patient and selection of a multiresistant endogenous flora that will influence antibacterial policy of that ICU in the future.
In the critically ill patient, isolation of multiresistant pathogens may occur in the following circumstances:[57]
-
individual isolation in a patient at risk (prolonged ICU stay, previous use of various combinations of broad spectrum antibacterials, high severity score). In this situation, multiresistant strains, such MRSA, S. maltophilia, P. aeruginosa, Candida albicans or other species of fungi, are frequently identified in the last phase of the clinical course of the patient. The presence of these pathogens is a marker of severity, although it has a low effect on the final outcome for the patient or on the antibiotic policy of the ICU
-
isolation of one or more resistant strains of the same species in the form of an epidemic outbreak (A. baumannii, K. pneumoniae containing ESBL, E. faecium, MRSA). As these strains form part of the environment of the ICU, patients are colonised rapidly, independently of their degree of severity. This form of presentation has an important impact on the antibiotic policy of the ICU and a major effect on the clinical course of patients with intermediate degrees of illness severity
-
detection of resistance of the original strain, particularly during treatment with cephalosporins, in relation to the development of antibacterial-induced inactivating enzymes (β-lactamases), especially in the case of Enterobacteriaceae and P. aeuruginosa. This event is associated with failure of antimicrobial therapy and an increase in mortality. In the case of persistence, it is necessary to restrict the use of third generation cephalosporins. The importance of these multiresistant pathogens varies between different countries, whereas vancomycin-resistant Enterococcus spp. (VRE) are mainly responsible for outbreaks in ICUs in the US. Enterobacteriaceae containing ESBL and A. baumannii are particularly frequent in European ICUs.
6. Antimicrobial Policy in the ICU
Prevention of the development of multiresistant pathogens is the main goal of the antimicrobial policy of an ICU. Although a series of general strategies to reduce the presence of multiresistant pathogens have been proposed,[58,59] the implementation of these recommendations in ICUs requires the cooperation of a member of the intensive care team who is an expert in infection diseases. Recommendations allowing for better use of antimicrobials in ICUs[60] are shown in table VII.
Antimicrobial policy in ICUs is based on the preparation and implementation of protocols for different types of infections in the critically ill patient. All specialists involved in the prevention and treatment of infections should participate in the development of guidelines, although the intensive care specialist should be responsible for implementing recommendations and for auditing the fulfillment of guidelines in daily practice.
Information generated by Services of Clinical Microbiology is the basis of directed therapy and the cornerstone of empirical therapy. Early awareness of pathogens responsible for a particular infection and/or their susceptibility patterns allows and facilitates the use of antimicrobials in a directed manner. Moreover, protocols of empirical therapy should be modified according to periodical information of pathogens that predominate most significantly in recovered samples and the results of susceptibility testing. Collaboration with the ICU can be enhanced by holding joint meetings presenting data on changes of infection indicators and resistance markers, and discussing therapeutic strategies in relation to the development of multiresistant strains. Some hospitals have available ‘guidelines for the use of antimicrobial agents’ with information on rational use of antimicrobials for each type of prophylaxis or infection reached by consensus. Intensive care physicians responsible for control and surveillance of infections and the use of antimicrobials should participate in the preparation of therapeutic protocols for the most frequent infections seen in the ICU.[61]
Restrictive policies in the area of antimicrobial use vary from strict prohibition of the use of a particular antimicrobial to the requirement of a written justification for the use of some antimicrobials or of a previous consultation with an expert. These policies, widely implemented in some countries, are associated with a low level of adherence among physicians and an excessive amount of bureaucracy that in the ICUs may led to nonadherence to guidelines or to a lack of rigorousness in the information requested.[62]
The development of computer-assisted management programs for antimicrobial use has given a great impulse to the rational control of antimicrobials and to the possibility of eliminating restrictive strategies.[63,64] However, despite these programmes, good communication with staff responsible for infectious diseases in each hospital service, especially with those in ICUs, ensures not only a correct use of antimicrobial agents but also the introduction of early changes when incorrect antimicrobial regimens are detected.
References
Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European prevalence of infection in intensive care (EPIC) study. JAMA 1995; 274: 639–44
Vaque J, Rossello J, Trilla A, et al. Nosocomial infections in Spain: results of 5 nationwide serial prevalence surveys (EPINE Project, 1990 to 1994). Infect Control Hosp Epidemiol 1996; 17: 293–7
Insausti J, Alvarez-Lerma F, de la Cal MA, et al. Antibiotic usage patterns and trends in Spanish Intensive Care Unit (ICUs) [abstract]. Intensive Care Med 1997; 23 Suppl. 1: S110
Feeley TW, du Moulin GC, Hedley-Whyte J, et al. Aerosol polymyxin and pneumonia in serious ill patients. N Engl J Med 1975; 293: 471–5
Gaman W, Cates C, Snelling CF, et al. Emergence of gentamicin- and carbenicillin-resistant Pseudomonas aeruginosa in a hospital environment. Antimicrob Agents Chemother 1976; 9: 474–80
Vogel F, Knothe H. Changes in aerobic faecal bacterial flora of severely ill patients during antibiotic treatment. Klin Wochenschr 1985; 63: 1174–9
Dworzack DL, Pugsley MP, Sanders CC, et al. Emergence of resistance in Gram-negative bacteria during therapy with extended-spectrum cephalosporins. Eur J Clin Microbiol 1987; 6: 456–9
Webb CH. Antibiotic resistance associated with selective decontamination of the digestive tract [editorial]. J Hosp Infect 1992; 22: 1–5
Nardi G, Valentinis U, Proietti A, et al. Epidemiological impact of prolonged systematic use of topical SDD on bacterial colonization of the tracheobronchial tree and antibiotic resistance. A 3 year study. Intensive Care Med 1993; 19: 273–8
Kunin CM. Problems in antibiotic usage. In: Mandell GL, Douglas RG, Bennet JE, editors. Principles and practice of infections diseases. New York: Churchill Livingstone, 1990; 427–33
Roder BL, Nielsen SL, Magnussen P, et al. Antibiotic usage in an intensive care unit in a Danish university hospital. J Antimicrob Chemother 1993; 32: 633–42
Mosdell DM, Morris DM, Voltura A, et al. Antibiotic treatment for surgical peritonitis. Ann Surg 1991; 214: 543–9
Luna CM, Vujacich P, Niederman MS, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 1997; 111: 676–85
Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among criticlly ill patients. Chest 1999; 115: 462–74
Polk R. Optimal use of modern antibiotics: emerging trends. Clin Infect Dis 1999; 29: 264–74
Lacy MK, Nicolau DP, Nightingale CH, et al. The pharmacodynamics of aminoglycosides. Clin Infect Dis 1998; 27: 23–7
Lacy MK, Lu W, Xu X, et al. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin againts Streptococcus pneumoniae in an in vitro model of infection. Antimicrob Agents Chemother 1999; 43: 672–7
Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis 1998; 27: 10–22
Fink MP, Snydman DR, Niederman MS, et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with impipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother 1994; 38: 547–57
Silver DR, Cohen IL, Weinberg PF. Recurrent Pseudomonas aeruginosa pneumonia in a intensive care unit. Chest 1992; 101: 194–8
Crouch Brewer S, Wunderink RG, Jones CB, et al. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 1996; 109: 1019–29
Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis 1990; 142: 523–8
Alvarez-Lerma F, ICU-Acquired Pneumonia Study Group. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med 1996; 22: 387–94
Barriere SL, Lowry SF. An overview of mortality risk prediction in sepsis. Crit Care Med 1995; 23: 376–93
Palomar M, Alvarez-Lerma F, de la Cal MA, et al. ICU infections in Spain. Time trends of etiology and resistances from 1994 to 1998 [abstract]. Intensive Care Med 1999; 25: 163
Rello J, Rodriguez R, Jubert P, et al. Severe community-acquired pneumonia in the elderly: epidemiology and prognosis. Clin Infect Dis 1996; 23: 723–8
Valles J, Leon C, Alvarez-Lerma F, et al. Nosocomial bacteremia in critically ill patients: a multicenter study evaluating epidemiology and prognosis. Clin Infect Dis 1997; 24: 387–95
Alvarez-Lerma F, Palomar M, Martinez-Pellus A, et al. Etiology and diagnostic techniques in Intensive care acquired pneumonia. Clin Intensive Care 1997; 8: 164–70
Carmeli Y, Troillet N, Eliopoulos GM, et al. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risk associated with different antipseudomonal agents. Antimicrob Agents Chemother 1999; 43: 1379–82
Damjanovic V, van Saene HKF. The value of surveillance cultures in neonatal intensive care units. In: van Saene HKF, Silvestri L, de la Cal MA, editors. Infection control in the intensive care unit. Berlin: Springer-Verlag, 1998; 316–22
van Saene HKF, Damjanovic V, Silvestri L, et al. Classification on infections. In: van Saene HKF, Silvestri L, de la Cal MA, editors. Infection control in the intensive care unit. Berlin: Springer-Verlag, 1998: 17–28
Viladrich PF, Gudiol F, Linares J, et al. Evaluation of vancomycin for therapy of adult pneumococcal meningitis. Antimicrob Agents Chemother 1991; 35: 2467–72
Pennington JE. Penetration of antibiotics into respiratory secretions. Rev Infect Dis 1981; 3: 67–73
Büchler M, Malfertheiner P, Friess H, et al. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology 1992; 103: 1902–8
Pelaez T, Bouza E. Actividad antianaerobicida de los carbapenemicos. Enferm Infecc Microbiol Clin 1997; 15 Suppl. 1:8–13
Pruul H, McDonald PJ. Damage to bacteria by antibiotics in vitro and its relevance to antimicrobial chemotherapy: a historical perspective. J Antimicrob Chemother 1988; 21: 695–8
ter Braak EW, de Vries PJ, Bouter KP, et al. Once daily dosing regimen for aminoglycoside plus betalactam combination therapy of serious infections: comparative trial with netilmicin plus ceftriaxone. Am J Med 1990; 89: 58–66
Prins JM, Buller HR, Kuijper EJ, et al. Once versus thrice daily gentamicin in patients with serious infections. Lancet 1993; 341: 335–9
Nicolau DP, Freeman CD, Belliveau PP, et al. Experience with a once-daily aminoglycoside program administered to 2,184 adults patients. Antimicrob Agents Chemother 1995; 39: 650–5
Hatala R, Dinh T, Cook DJ. Once-daily aminoglycoside dosing in immunocompentent adults: a meta-analysis. Ann Intern Med 1996; 124: 717–25
Barza M, Ioannidis JPA, Cappelleri JC, et al. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ 1996; 312: 338–44
Rodvold KA, Danziger LH, Quinn JP. Single daily doses of aminoglycosides. Lancet 1997; 350: 1412
Visser LG, Arnouts P, van Furth R, et al. Clinical pharmacokinetics of continuous intravenous administration of penicillins. Clin Infect Dis 1993; 17:491–5
Hyatt JM, McKinnon PS, Zimmer GS, et al. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet 1995; 28: 143–60
Tessier PR, Nicolau DP, Onyeji CD, et al. Pharmacodynamics of intermitent- and continuous-infusion cefepime alone and in combination with once-daily tobramycin against Pseudomonas aeruginosa in an in vitro infection model. Chemotherapy 1999; 45: 284–95
Daenen S, de Vries-Hospers H. Cure of Pseudomonas aeruginosa infection in neutropenic patients by continuous infusion of ceftazidime [letter]. Lancet 1988; I: 937
Vinks AAT, Touw DJ, Heijerman HGM, et al. Pharmacokinetics of ceftazidime in adult cystic fibrosis during continuous infusion and ambulatory treatment at home. Ther Drug Monit 1994; 16: 341–8
Daenen S, Erjavec Z, Uges DR, et al. Continuous infusion of ceftazidime in febrile neutropenic patients with acute myeloid leukemia. Eur J Clin Microb Infect Dis 1995; 14: 188–92
Di Filippo A, DE Gaudio AR, Novelli A, et al. Continuous infusion of vancomycin in methicillin-resistant staphylococcus infection. Chemotherapy 1998; 44: 63–8
James JK, Palmer SM, Levine DP, et al. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40: 696–700
Leder K, Turnidge JD, Korman TM, et al. The clinical efficacy of continuous-infusion flucloxacillin in serious staphylococal sepsis. J Antimicrob Chemother 1999; 43: 113–8
Makhoul IR, Mezbach D, Lichtig C, et al. Antibiotic treatment of experimental Pseudomonas aeruginosa pneumonia in guinea pigs: comparison of aerosol and systemic administration. J Infect Dis 1993: 168: 1296–9
Brown RB, Kruse JA, Counts GW, et al. Double-blind study of endotracheal tobramycin in the treatment of Gram-negative bacterial pneumonia. Antimicrob Agents Chemother 1990; 34: 269–72
Stoutenbeek CP, van Saene HKF, Miranda DR, et al. Nosocomial gram-negative pneumonia in critically ill patients. A 3-year experience with a novel therapeutic regimen. Intensive Care Med 1986; 12:419–23
Begg EJ, Barclay ML, Kirkpatrick CJM. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol 1999; 47: 23–30
Slattery JT. A pharmacokinetic model-independent approach for estimating dose required to give derived steady-state trough concentrations of drug in plasma. J Pharmacokinet Biopharm 1980; 8: 105–10
Alvarez Lerma F. Impacto de las resistencias bacterianas sobre la politica antibiótica. Med Intensiva 1998; 22: 17–23
Yates RR. New intervention strategies for reducing antibiotic resistance. Chest 1999; 115; 24S–7S
Burgess DS. Pharmacodynamic principles of antimicrobial therapy in the prevention of resistance. Chest 1999; 115: 19S–23S
Alvarez Lerma F, Palomar Martinez M. Decalogo de normas para la utilización de antibióticos en pacientes críticos. Med Intensiva 2000; 24: 69–77
Drobnic L, Grau S, Salas E, et al. Guía terapéutica antimicrobiana, 1995. Madrid. Consorci Sanitari de Barcelona. IMAS, 1995
Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow practice guidelines? A framework for improvement. JAMA 1999; 282: 1458–65.
Grau S, Monterde J, Carmona A, et al. Monitoring of antimicrobial therapy by an integrated computer program. Pharm World Sci 1999; 21: 152–7
Evans RS, Pestotnik SL, Classen DC, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998; 338: 232–8
Acknowledgements
We thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Álvarez-Lerma, F., Palomar, M. & Grau, S. Management of Antimicrobial Use in the Intensive Care Unit. Drugs 61, 763–775 (2001). https://doi.org/10.2165/00003495-200161060-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200161060-00005