Summary
Abstract
Raloxifene is a selective estrogen receptor modulator that partially mimics the effects of estrogens in bone and the cardiovascular system, while functioning as an antiestrogen in endometrial and breast tissue.
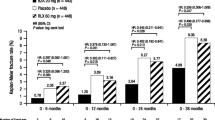
In randomised placebo-controlled studies involving postmenopausal women or patients with osteoporosis, raloxifene 60 to 150 mg/day was effective in increasing bone mineral density (BMD) over 12-to 36-month periods. At the 60 mg/day recommended dosage, increases of 1.6 to 3.4%, 0.9 to 2.3% and 1.0 to 1.6% were reported in lumbar spine, femoral neck and total hip, respectively, versus ≤0.5% with placebo.
Raloxifene 60 or 120 mg/day decreased the risk of vertebral fractures over a 36-month period in postmenopausal patients with osteoporosis. Significant reductions in radiographic fracture risk versus placebo (30 and 50%) occurred regardless of whether patients had existing fractures at baseline. Although raloxifene did not affect the overall incidence of nonvertebral fractures, a reduction in the incidence of ankle fracture was reported in comparison with placebo. In postmenopausal women, raloxifene 60 mg/day significantly reduced serum levels of total and low density lipoprotein cholesterol from baseline, compared with placebo. High density lipoprotein cholesterol and triglyceride levels were unaffected.
Raloxifene 60 or 120 mg/day reduced the risk of invasive breast cancer by 76% during a median of 40 months’ follow-up in postmenopausal patients with osteoporosis and no history of breast cancer. A relative risk reduction of 90% was reported for estrogen-receptor positive invasive breast cancers; estrogen-receptor negative cancer risk was unaffected by raloxifene.
Raloxifene was generally well tolerated in clinical trials at dosages up to 150 mg/day. Adverse events thought to be related to raloxifene treatment were hot flushes and leg cramps. Venous thromboembolism was the only serious adverse event thought to be related to raloxifene treatment and a relative risk of 3.1 compared with placebo treatment was reported in patients with osteoporosis. Vaginal bleeding occurred in ≤6.4% of raloxifene-treated women but was reported by 50 to 88% of those receiving estrogens or hormone replacement therapy (HRT). Raloxifene treatment was not associated with stimulatory effects on the endometrium.
Conclusions: Raloxifene significantly increases BMD in postmenopausal women and reduces vertebral fracture risk in patients with osteoporosis. In clinical trials, raloxifene was generally well tolerated compared with placebo and HRT, although its propensity to cause hot flushes precludes use in women with vasomotor symptoms. In particular, the lack of stimulatory effects on the endometrium and the reduction in invasive breast cancer incidence indicate raloxifene as an attractive alternative to HRT for the management of postmenopausal osteoporosis.
Pharmacodynamic Properties
Raloxifene is a selective estrogen receptor modulator that partially mimics the effects of estrogens in bone and the cardiovascular system and has estrogen antagonist properties in endometrial and breast tissue.
Mechanism of Action: Raloxifene appears to influence gene transcription, via the intermediary of estrogen receptors (ER), through interactions with the estrogen response element (ERE) and a distinct DNA target, the raloxifene response element (RRE). However, the precise mechanism of action of this agent remains to be elucidated.
Bone Remodelling: Raloxifene modulates bone cell homeostasis in vitro, through actions on the proliferation and activity of osteoclasts and osteoblasts. Effects include inhibition of osteoclast pit formation and increases in the expression of bone matrix proteins such as alkaline phosphatase, osteonectin, osteocalcin and collagen.
In postmenopausal women and patients with osteoporosis, raloxifene 30 to 150 mg/day consistently and significantly reduced serum and urinary markers of bone turnover compared with placebo treatment. The effects of raloxifene 60 mg/day on bone turnover were less than those of conjugated equine estrogens 0.625 mg/day in a 6-month study. Raloxifene 60 mg/day plus alendronate 10 mg/day appeared to have greater effects on markers of bone turnover over a 12-month period than either agent alone.
Histomorphometric analysis of iliac crest biopsies indicated that 6 months’ treatment with raloxifene 60 mg/day maintained bone architecture and had no adverse effects on mineralisation rate or bone quality in postmenopausal women. These effects were similar to those of conjugated equine estrogens 0.625 mg/day. Cardioprotective Potential: Antiatherogenic properties have been reported in animal models and in in vitro studies.
In postmenopausal women, raloxifene 30 to 150 mg/day was consistently more effective than placebo in reducing serum levels of total and low density lipoprotein cholesterol. Effects were apparent within 3 to 6 months of treatment onset and were maintained for the remainder of 12 and 24-month treatment periods. Unlike HRT which significantly increased triglyceride levels compared with placebo, raloxifene had no effect on this parameter. However, in contrast to HRT, raloxifene did not elevate high density lipoprotein cholesterol levels.
Significant reductions in serum levels of homocysteine and tumour necrosis factor-α were reported during raloxifene treatment and these effects were similar to those of estrogens or HRT. In contrast with HRT, which reduced tissue plasminogen activator and plasminogen activator inhibitor-1 levels and increased C-reactive protein, raloxifene had no effect on these parameters. However, while placebo had no effect, raloxifene decreased levels of apolipoprotein B and fibrinogen.
Effects on Breast Tissue: Raloxifene has in vitro antiproliferative effects on human breast cancer cells and inhibits mammary carcinogenesis in animal models of breast cancer.
A total of 54 newly diagnosed breast cancers were reported during a median of 40 months of follow-up in a large (n = 7705) osteoporosis treatment study (representing >15 000 and >7400 patient-years of exposure to raloxifene (60 or 120 mg/day, pooled dosages) and placebo, respectively]. Treatment with raloxifene reduced the relative risk of developing any breast cancer by 65% compared with placebo treatmet and a 76% reduction in risk of invasive breast cancer was reported. The reduction in risk of invasive breast cancer was similar between 60 and 120 mg/day dosage groups. Additionally, raloxifene reduced the risk of estrogen-receptor positive breast cancers by 90% but did not affect estrogen-receptor negative cancer risk.
Endometrial Effects: In contrast to the increases in endometrial thickness or uterine volume observed during treatment with conjugated equine estrogens, hormone replacement therapy (HRT) and tamoxifen, stimulatory effects on the endometrium were not observed after up to 2 years’ treatment of postmenopausal women with raloxifene 60 or 150 mg/day. Moreover, endometrial histology was not altered by raloxifene at supratherapeutic dosages of 200 or 600 mg/day for 8 weeks.
Effects on the CNS: In randomised double-blind placebo-controlled studies of 1 or 3 years’ duration involving postmenopausal women, raloxifene did not affect cognitive function, and no treatment differences in measures of memory, anxiety or depression were reported between raloxifene 60 to 150 mg/day or placebo.
Pharmacokinetic Properties
Raloxifene is rapidly absorbed from the gastrointestinal tract (approximately 60%) and undergoes extensive first-pass glucuronidation. Peak plasma concentrations (Cmax) of raloxifene and its metabolites typically occur 6 hours after oral administration and a median absolute bioavailability of 2% has been reported. After multiple doses, Cmax and area under the plasma concentration-time curve (AUC) values of raloxifene were 1.36 µg/L per mg/kg and 24.2 µg · h/L per mg/kg.
Raloxifene is extensively distributed after oral administration and an apparent volume of distribution of 2348 L/kg has been reported. In vitro, raloxifene is >95% bound to human plasma proteins. The plasma elimination half-life of raloxifene is 27.7 hours.
Raloxifene is metabolised by glucuronidation and unchanged drug accounts for 1% of circulating concentrations. Excretion is predominantly via the faecal route and occurs within 5 days of administration; approximately 5% of the administered dose is excreted in urine as glucuronide conjugates.
Coadministration of warfarin with raloxifene produced small but significant reductions in clearance and volume of distribution of the warfarin enantiomers. Maximum prothrombin response and prothrombin time AUC were reduced by 10 and 8%, respectively. Warfarin administration did not affect the pharmacokinetic properties of raloxifene. A 60% reduction in the absorption and enterohepatic recirculation of raloxifene was reported after a single dose of the anion exchange resin cholestyramine.
Therapeutic Use
During the clinical trials programme for raloxifene, therapeutic efficacy in terms of prevention of bone loss in postmenopausal women and treatment of postmenopausal patients with osteoporosis has been evaluated on the basis of changes in bone mineral density (BMD) and fracture incidence. Raloxifene, at dosages of 60 to 150 mg/day, was administered as an oral once daily regimen.
In postmenopausal women and patients with osteoporosis enrolled in randomised multicentre trials, raloxifene (generally in the presence of calcium and vitamin D3 supplementation) consistently increased lumbar spine, femoral neck, otal hip and total body BMD relative to baseline values over 12-to 36-month treatment periods. Significant increases versus placebo in BMD were evident within 12 months and were maintained for the remainder of treatment. At the 60 mg/day therapeutic dosage, increases of 1.6 to 3.4%, 0.9 to 2.3% and 1 to 1.6% were reported in the lumbar spine, femoral neck and total hip, respectively. Mean BMD values were reduced compared with baseline or increased by ≤0.5% in individuals receiving placebo.
In a 12-month study, treatment with raloxifene 60 mg/day plus alendronate 10 mg/day significantly increased lumbar spine and proximal femur BMD to a greater extent than either agent alone or placebo. Alendronate tended to increase BMD at both sites to a greater extent than raloxifene, although this difference was not significant.
In a large randomised double-blind placebo-controlled study involving 7705 patients with postmenopausal osteoporosis, the relative risk of vertebral fractures was reduced by raloxifene 60 or 120 mg/day. Over a 36-month treatment period, significant reductions in radiographic fracture risk (30 and 50%) relative to placebo occurred regardless of whether patients had pre-existing fractures at baseline.Moreover, the risk of clinical (painful) vertebral fractures was reduced by 60% during raloxifene treatment. Raloxifene significantly reduced the incidence of ankle fracture compared with placebo treatment (0.7 vs 1.1%, p < 0.05), although there was no difference between groups for hip, wrist or total nonvertebral fractures.
Tolerability
Raloxifene at dosages up to 150 mg/day has been generally well tolerated in clinical trials in postmenopausal women. Adverse events thought to be related to raloxifene treatment were hot flushes and leg cramps. No clinically important changes in haematological, renal or hepatic laboratory variables were observed during 3-year raloxifene treatment.
Hot flushes occurred in 24.6% of postmenopausal women treated for up to 30 months with raloxifene, compared with 18.3% receiving placebo (p < 0.001) and were more frequent (p < 0.05) during treatment with raloxifene than with HRT or conjugated equine estrogens. In the large osteoporosis treatment study, 9.7 and 11.6% of patients receiving raloxifene 60 and 120 mg/day, respectively, experienced hot flushes, compared with 6.4% in the placebo group. Most episodes of hot flush were mild or moderate in intensity and tended to occur more commonly in the first 6 months of treatment. The occurrence of other vasomotor symptoms such as daytime and night sweats and sleep disorders did not differ between raloxifene, placebo, HRT and estrogen treatment.
Leg cramps occurred more commonly in individuals receiving raloxifene than in the placebo group (5.5 vs 1.9%), although the incidence of this event was not significantly different between raloxifene, HRT and estrogen treatment. Similarly, leg cramps occurred in 7.0, 6.9 and 3.7% of patients receiving raloxifene 60 or 120 mg/day or placebo, respectively, in the large osteoporosis treatmentstudy. In this trial, the incidence of peripheral oedema was also more common (p < 0.01) with raloxifene treatment (5.2 and 6.5%) than with placebo (4.4%). Breast pain was rare during raloxifene treatment and occurred less frequently than during treatment with HRT or estrogens.
Few episodes of vaginal bleeding occurred during treatment with raloxifene (<6.4%) and the incidence was similar to that reported among individuals receiving placebo. In contrast, vaginal bleeds were reported by 50 to 88.5% of postmenopausal women receiving estrogens or HRT. In a 3-year study involving 7705 patients, endometrial carcinoma occurred in 4, 2 and 4 patients receiving raloxifene 60 or 120 mg/day or placebo, respectively.
The only serious adverse events considered related to raloxifene treatment were venous thromboembolic episodes. Although the incidence was low (1% in each of 60 and 120 mg/day groups vs 0.3% with placebo) a relative risk of thromboembolic events of 3.1 was reported. The greatest risk of thromboembolism occurred within the first 4 months of therapy.
Dosage and Administration
Oral raloxifene is indicated for the treatment and prevention of postmenopausal osteoporosis at a dosage of 60 mg/day, and may be administered without respect to mealtimes. Supplemental calcium and/or vitamin D should be added to the diet if the daily intake is inadequate.
Raloxifene is contraindicated in patients with known hypersensitivity to raloxifene and in those with a history of thromboembolic events. Raloxifene should be discontinued ≥72 hours prior to and during prolonged immobilisation, and should be resumed only after the patient is fully ambulatory.
Concomitant use of raloxifene with systemic estrogens or cholestyramine is not recommended and raloxifene should be used with caution in patients taking highly protein-bound drugs such as diazepam, diazoxide and lidocaine (lignocaine). In patients taking concomitant warfarin or other coumarin derivatives, prothrombin times should be closely monitored when starting or stopping raloxifene treatment.
Similar content being viewed by others
References
World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. WHO Technical Report Series 1994; 843: 1–129
Sato M, Grese TA, Dodge JA, et al. Emerging therapies for the prevention or treatment of postmenopausal osteoporosis. J Med Chem 1999; 42(1): 1–24
Kiel DP, Felson DT, Anderson JJ, et al. Hip fracture and the use of estrogens in postmenopausal women. The Framingham Study. N Engl J Med 1987; 317: 1169–74
Cauley JA, Seeley DG, Ensrud K, et al. Estrogen replacement therapy and fractures in older women. Ann Intern Med 1995; 122: 9–16
Lufkin EG, Wahner HW, O’Fallon WM, et al. Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med 1992; 117: 1–9
Ettinger B, Genant HK, Cann CE. Long-term estogen replacement therapy prevents bone loss and fractures. Ann Intern Med 1985; 102: 319–24
Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurse’s Health Study. N Engl J Med 1991; 325: 756–62
Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992; 117: 1016–37
Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med 1997; 336: 1769–75
Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998; 280: 605–13
Beresford SAA, Weiss NS, Voigt LF, et al. Risk of endometrial cancer in relation to use of oestrogen combnined with cyclic progestagen therapy in postmenopausal women. Lancet 1997; 349: 458–61
Brinton LA, Hoover RN. Estrogen replacement therapy and endometrial cancer risk: unresolved issues. The Endometrial Cancer Collaborative Group. Obstet Gynecol 1993; 81: 265–71
Voigt LF, Weiss NS, Chu J, et al. Progestagen supplementation of exogenous oestrogens and risk of endometrial cancer. Lancet 1991; 338: 274–7
Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 1995; 332: 1589–93
Steinberg KK, Thacker SB, Smith SJ, et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 1991; 265: 1985–90
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52705 women with breast cancer and 108411 women without breast cancer. Lancet 1997; 350: 1047–59
Torgerson DJ, Donaldson C, Russell IT, et al. Hormone replacement therapy: compliance and cost after screening for osteoporosis. Eur J Obstet Gynecol Reprod Biol 1995; 59: 57–60
Berman RS, Epstein RS, Lydick EG. Compliance of women taking estrogen replacement therapy. J Womens Health 1996; 5: 213–20
Gustafsson JA. Therapeutic potential of selective estrogen receptor modulators. Curr Opin Chem Biol 1998; 2: 508–11
Bryant HU, Glasebrook AL, Yang NN, et al. A pharmacological review of raloxifene. J Bone Miner Metab 1996; 14: 1–9
Macgregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev 1998; 50: 151–96
Kauffman RF, Bryant HU, Yang N, et al. Preventing postmenopausal osteoporosis: an update on raloxifene. Drug News Perspect 1999; 12: 223–33
Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 1996; 88: 1529–42
Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-l Study. J Natl Cancer Inst 1998; 90: 1371–88
Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. JAMA 1999; 281: 2189–97
Prevention study of tamoxifen and raloxifene (STAR) in postmenopausal women at increased risk for invasive breast cancer [online]. CancerNet Protocols Database, 1999 Jul. Available from URL: http://cancernet.nci.nih.gov/cgibin/srchcgi.exe Accessed Apr 19 2000]
Barrett-Connor E, Wenger NK, Grady D, et al. Coronary heart disease in women, randomized clinical trials, HERS and RUTH. Maturitas 1998; 31: 1–7
Sato M, Glasebrook AL, Bryant HU. Raloxifene: a selective estrogen receptor modulator. J Bone Miner Metab 1994; 12 Suppl. 2: S9-S20
Hol T, Cox MB, Bryant HU, et al. Selective estrogen receptor modulators and postmenopausal women’s health. J Women Health 1997; 6: 523–31
Khovidhunkit W, Shoback DM. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med 1999; 130: 431–9
Yang NN, Venugopalan M, Hardikar S, et al. Identification of an estrogen response element activated by metabolites of 17-beta-estradiol and raloxifene. Science 1996; 273: 1222–5
Gize EA, Venugopalan M, Glasebrook AL, et al. Characterization of raloxifene binding and transactivation properties of the estrogen receptor-beta (ERβ) [abstract S428]. J Bone Miner Res 1927 12 Suppl.: S460
Yang NN, Bryant HU, Hardikar S, et al. Estrogen and raloxifene stimulate transforming growth factor-beta 3 gene expression in rat bone: a potential mechanism for estrogen-or raloxifene-mediated bone maintenance. Endocrinology 1996; 137: 2075–84
Krane SM, Holick MF. Metabolic bone disease. In: Fauci AS, Braunwald E, Isselbacher KJ et al, editors. Harrisons principles of internal medicine. 14th ed. vol. 2. New York: McGraw-Hill, 1999: 2247–53
Migliaccio S, Teti A, Taranta A, et al. Raloxifene modulates osteoclastogenesis and bone resorption in vitro [abstract]. Abstracts of the 3rd International Symposium on Women’s Health and Menopause; 1998 Jun 13–16, Florence: 19
Migliaccio S, Taranta A, Teti A, et al. Raloxifene directly modulates bone cell activity in vitro [abstract]. Bone 1998; 23 Suppl.: 608
Lengner C, Green J, Bodine PVN, et al. Selective modulation of osteoblast growth and differentiation parameters by antiestrogens [abstract]. Bone 1998; 23(5) Suppl.: S206
Fournier B, Häring S, Kaye AM, et al. Stimulation of creatine kinase specific activity in human osteoblast and endometrial cells by estrogens and anti-estrogens and its modulation by calciotropic hormones. J Endocrinol 1996; 150: 275–85
Evans G, Bryant HU, Magee D, et al. The effects of raloxifene on tibia histomorphometry in ovariectomized rats. Endocrinology 1994; 134: 2283–8
Evans GL, Bryant HU, Magee DE, et al. Raloxifene inhibits bone turnover and prevents further cancellous bone loss in adult ovariectomized rats with established osteopenia. Endocrinology 1996; 137: 4139–44
Turner CH, Sato M, Bryant HU. Raloxifene preserves bone strength and bone mass in ovariectomized rats. Endocrinology 1994; 135: 2001–5
Bryant HU, Turner CH, Frolik CA, et al. Long term effects of raloxifene (139481 HCl) on bone, cholesterol and uterus in ovariectomized rats [abstract 125]. Bone 1995; 16 Suppl.: 116S
Delmas PD, Bjarnason NH, Mitlak B, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations and uterine endometrium in postmenopausal women. N Engl J Med 1997; 337(23): 1641–7
Bjarnason NH, Delmas PD, Mitlak BH. Raloxifene maintains favourable effects on bone mineral density, bone turnover and serum lipids without endometrial stimulation in postmenopausal women. 3-year study results [abstract OR22]. Osteoporos Int 1998; 8 Suppl. 3: 11
Gunness M, Prestwood K, Lu Y, et al. Histomorphometric, bone marker, and bone mineral density response to raloxifene HCl and premarin in postmenopausal women [abstract no. OR4-2]. The Endocrine Society 79th Annual Meeting Programme, 1997, Jun 11–14: Minneapolis, USA. The Endocrine Society Press, Bethesda (MD): 67
Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 1999; 282: 637–45
Johnell O, Scheele W, Lu Y, et al. Effects of raloxifene (RLX), alendronate (ALN) and RLX + ALN on bone mineral density (BMD) and biochemical markers of bone turnover in postmenopausal women with osteoporosis [abstract]. In: J Bone Miner Res 1999: 14 Suppl. 1: S157
Lufkin EG, Whitaker MD, Nickelsen T, et al. Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res 1998; 13: 1747–54
Meunier PJ, Vignot E, Garnero P, et al. Treatment of postmenopausal women with osteoporosis or low bone density with raloxifene. Osteoporos Int 1999; 10(4): 330–6
Bryant HU, Cole HW, Rowley ER, et al. Raloxifene and LY117018 prevent bone loss induced by an LH-RH agonist, without estrogen-like stimulation of the uterus [abstract]. 10th International Congress of Endocrinology; 1996 Jun 12–15; San Francisco (CA), 572
Prestwood KM, Gunness M, Muchmore DB, et al. A comparison of the effects of raloxifene and estrogen on bone in postmenopausal women. J Clin Endocrinol Metab 2000; 85: 2197–202
Eli Lilly & Company. Evista: raloxifene hydrochloride prescribing information. Indianapolis (IN): Eli Lilly, 1999 Sep 30
Heaney RP, Draper MW. Raloxifene and estrogen: comparative bone-remodeling kinetics. J Clin Endocrinol Metab 1997; 82: 3425–9
Kauffman RF, Bensch WR, Roudebush RE, et al. Hypocholesterolemic activity of raloxifene (LY139481): pharmacological characterization as a selective estrogen receptor modulator. J Pharmacol Exp Ther 1997; 280: 146–53
Black LJ, Sato M, Rowley ER, et al. Raloxifene (LY139481 HCl) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest 1994; 93: 63–9
Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomised rats. FASEB J 1996; 10: 905–12
Frolik CA, Bryant HU, Black EC, et al. Time-dependent changes in biochemical bone markers and serum cholesterol in ovariectomized rats: effects of raloxifene HCl, tamoxifen, estrogen, and alendronate. Bone 1996; 18: 621–7
Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA 1998; 279: 1445–51
de Valk de Roo GW, Stehouwer CDA, Meijer P, et al. Both raloxifene and estrogen reduce major cardiovascular risk factors in healthy postmenopausal women. A 2-year, placebo-controlled study. Arterioscler Thromb Vasc Biol 1999; 19: 2993–3000
Walsh BW, Paul S, Wild RA, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 2000; 85: 214–8
Cox DA, Sashegyi A, Paul S, et al. Effects of raloxifene and hormone replacement therapy on markers of inflammation in healthy postmenopausal women [abstract 4538]. Circulation 1999; 100 Suppl. I: I-826
Bjarnason NH, Haarbo J, Byrjalsen I, et al. Raloxifene reduces atherosclerosis: studies of optimized raloxifene doses in ovariectomized, cholesterol-fed rabbits. Clin Endocrinol 2000; 52: 225–33
Steinberg D, Parthasarathy S, Carew TE, et al. Beyond cholesterol. Modifications of low density lipoprotein increase its atherogenicity. N Engl J Med 1998; 320: 915–24
Zuckerman SH, Bryan N. Inhibition of LDL oxidation and myeloperoxidase dependent tyrosyl radical formation by the selective estrogen receptor modulator raloxifene (LY139481 HCl). Atherosclerosis 1996; 126: 65–75
Wiernicki T, Glasebrook A, Phillips DL, et al. Estrogen and a novel tissue selective estrogen receptor modulator raloxifene directly modulate vascular smooth muscle cell functions: implications in the cardioprotective mechanism of estrogen [abstract 1615]. Circulation 1996; 94 Suppl. I: I-278
Short LL, Dodge JA, Glasebrook AL, et al. Comparison of a series of estrogens and serms regarding proliferation of a human breast cancer cell line [abstract]. Programs and Abstracts of the 81st Annual Meeting of the Endocrine Society; 1999 Jun 12–15; San Diego, CA: 186
Clemens JA, Bennett DR, Black LJ, et al. Effects of a new antiestrogen, keoxifene (LY156758), on growth of carcinogen-induced mammary tumors and on LH and prolactin levels. Life Sci 1983; 32: 2869–75
Anzano MA, Peer CW, Smith JM, et al. Chemoprevention of mammary carcinogenesis in the rat: combined use of raloxifene and 9-cis-retinoic acid. J Natl Cancer Inst 1996; 88: 123–5
Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res 1987; 47: 4020–4
Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol 2000; 95: 95–103
Fugère P, Scheele WH, Shah A, et al. Uterine effects of raloxifene in comparison with continuous-combined hormone replacement therapy in postmenopausal women. Am J Obstet Gynecol 2000; 182: 568–74
Boss SM, Huster WJ, Neild JA, et al. Effects of raloxifene hydrochloride on the endometrium of postmenopausal women. Am J Obstet Gynecol 1997; 177: 1458–64
Vardy MD, Cosman F, Heller D, et al. Short-term endometrial effects of raloxifene, tamoxifen, and premarin [abstract P-193]. Fertil Steril 1998; 70 Suppl. 1: S186-S187
Schneider LS, Finch CE. Can estrogens prevent neurodegeneration? Drugs Aging 1997; 11: 87–95
Yaffe C, Sawaya G, Lieberburg I, et al. Estrogen therapy in postmenopausal women. Effects on cognitive function and dementia. JAMA 1998; 279: 688–95
Haskell SG, Richardson ED, Horwitz RI, et al. The effect of estrogen replacement therapy on cognitive function in women: a critical review of the literature. J Clin Epidemiol 1997; 50: 1249–64
Nilsen J, Mor G, Naftolin F. Raloxifene induces neurite outgrowth in estrogen receptor positive PC12 cells. Menopause 1998; 5: 211–6
Lakshmanan MC, Bryant HU, Paul SM, et al. Central nervous system effects of raloxifene: evidence from animal models and controlled clinical trials in postmenopausal women [abstract P367]. Climacteric 1999; 2 Suppl. 1: 331
Nickelsen T, Lufkin EG, Riggs BL, et al. Raloxifene hydrochloride, a selective estrogen receptor modulator: safety assessment of effects on cognitive function and mood in postmenopausal women. Psychoneuroendocrinology 1999; 24: 115–28
Nickelsen T, Yaffe K, Krueger KA, et al. Effects of raloxifene on cognitive function in postmenopausal women without dementia [abstract 503]. Osteoporos Int 2000; 11 Suppl. 2: S191
Ni L, Allerheiligen SRB, Basson R, et al. Pharmacokinetics of raloxifene in men and postmenopausal women volunteers [abstract]. Pharm Res 1996; 13 Suppl.: S-430
Forgue ST, Rudy AC, Knadler MP, et al. Raloxifene pharmacokinetics in healthy postmenopausal women [abstract PPDM 8148]. Pharm Res 1996; 13 Suppl.: S429
Forgue ST, Rudy AC, Geiser J, et al. Interconversion pharmacokinetics of the selective estrogen receptor modulator raloxifene HCl [abstract]. Pharm Res 1997; 14 Suppl.: S-605
Knadler MP, Lantz RJ, Gillespie TA, et al. The disposition and metabolism of 14C-labeled raloxifene in humans [abstract]. Pharm Res 1995; 12 Suppl.: S-372
Skerjanec A, Miller JW, Basson R, et al. Pharmacokinetic and pharmacodynamic interaction study between raloxifene and warfarin [abstract]. Pharm Res 1997; 14 Suppl.: S-561-2
Reginster JY, Compston JE, Jones EA, et al. Recommendations for the registration of new chemical entities used in the prevention and treatment of osteoporosis. Calcif Tissue Int 1995; 57: 247–50
Bonjour J-P, Ammann P, Rizzoli R. Importance of preclinical studies in the development of drugs for treatment of osteoporosis: a review related to the 1998 WHO guidelines. Osteoporos Int 1999; 9(5): 379–93
Food and Drug Administration. Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis. Division of Metabolic and Endocrine Drug Products: Rockville,(MD). 1994
Ross PD, Davis JW, Epstein RS, et al. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991; 114: 919–23
Melton III LJ, Atkinson EJ, O’Fallon WM, et al. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res 1993; 8: 1227–33
Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fracture. Lancet 1993; 341: 72–5
Consensus Development Conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993; 94: 646–50
Houpt LS, Minshall DE, Shen W, et al. Quality of life results: randomized clinical trial of a selective estrogen receptor modulator, raloxifene, and hormone replacement therapy [in FC801] [abstract]. Proceedings of the XVth FIGO, 1997, 12
Stovall DW, Walsh BW, Melchione TE, et al. Raloxifene effects on quality of life in healthy postmenopausal women compared to unopposed estrogen and placebo, at 3-month and 12-month periods [abstract P-110]. Fertil Steril 1999; 72 Suppl. 1: S126
Pavo I, Masanauskaite D, Rojinskaya L, et al. Effects of raloxifene (RLX) vs. placebo (PL) on bone mineral density (BMD) in postmenopausal women in the absence of calcium supplementation [abstract]. JBone Miner Res 1999: 14 Suppl. 1:S410
Eastell R. Treatment of postmenopausal osteoporosis. N Engl J Med 1998; 338: 736–46
Lord S, Lu Y, Lakshamanan M, et al. Raloxifene does not affect neuromuscular function or the incidence of falls in postmenopausal women with osteoporosis [abstract P-53]. Menopause 1999; 6: 347–8
Houpt LS, Minshall ME, Shen W, et al. Study completer analysis of quality of life results from a clinical trial of a selective estrogen receptor modulator, raloxifene, and hormone replacement therapy [abstract 17]. Calcif Tissue Int 1997; 61: 503
Davies GC, Huster WJ, Lu Y. Adverse events reported by postmenopausal women in controlled trials with raloxifene. Obstet Gynecol 1999; 93: 558–65
Cohen FJ, Lu Y. Characterization of hot flashes reported by healthy postmenopausal women receiving raloxifene or placebo during osteoporosis prevention trials. Maturitas 2000; 34: 65–73
Consensus Development Statement. Who are candidates for the prevention and treatment of osteoporosis? Osteoporos Int 1997; 7: 1–6
Melton III LJ, Chrischilles EA, Cooper C, et al. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7: 1005–10
Jones G, Nguyen T, Sambrook PN, et al. Symptomatic fracture in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES). Osteoporos Int 1994; 4: 277–82
Marottoli RA, Berkman LF, Cooney LM. Decline in physical function following hip fracture. J Am Geriatr Soc 1992; 40: 861–6
Cummings SR, Phillips SL, Wheat ME, et al. Recovery of function after hip fracture. The role of social supports. J Am Geriatr Soc 1988; 36: 801–6
Ross PD. Osteoporosis. Frequency, consequences and risk factors. Arch Intern Med 1996; 156: 1399–411
Schürch MA, Rizzoli R, Mermillod B, et al. A prospective study on socioeconomic aspects of fracture of the proximal femur. J Bone Miner Res 1996; 11: 1935–42
Gold DT. The climical impact of vertebral fractures: quality of life in women with osteoporosis. Bone 1996; 18 Suppl. 3: 185S-189S
Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999; 353: 878–82
Meunier PJ, Delmas PD, Eastell R, et al. Diagnosis and management of osteoporosis in postmenopausal women: clinial guidelines. Clin Ther 1999; 21: 1025–44
Golden BD. The prevention and treatment of osteoporosis. Arthritis Care Res 1998; 11(2): 124–34
Francis RM. Management of established osteoporosis. Br J Clin Pharmacol 1998; 45(2): 95–9
Ettinger B, Genant HK, Cann CE. Postmenopausal bone loss is prevented by treatment with low-dosage estrogen with calcium. Ann Intern Med 1987; 106: 40–5
Schneider DL, Barrett-Connor EL, Morton DJ. Timing of postmenopausal estrogen for optimal bone mineral density. The Rancho Bernardo Study. JAMA 1997; 277: 543–7
Lindsay R, Hart DM, MacLean A, et al. Bone response to termination of oestrogen treatment. Lancet 1978; I(8078): 1325–7
Michaëlsson K, Baron JA, Farahmand BY, et al. Hormone-replacement therapy and risk of hip fracture: population based case-control study. The Swedish Hip Fracture Study Group. BMJ 1998; 316: 1858–63
Utian WH, Schiff I. NAMS-Gallup survey on women’s knowledge, information sources, and attitudes to menopause and hormone replacement therapy. Menopause 1994; 1: 39–48
Mitlak BH, Cohen FJ. In search of the optimal long-term female hormone replacement: the potential of selective estrogen receptor modulators. Horm Res 1997; 48: 153–63
Beardsworth SA, Purdie DW, Kearney CE. Selective oestrogen receptor modulation: an alternative to conventional oestrogen. Curr Obstet Gynaecol 1998; 8(2): 96–101
Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results form the Fracture Intervention Trial. JAMA 1998; 280: 2070–82
Black DM, Cummings SR, Karpf DB, et al. Randomised trial of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348: 1535–41
Col NF, Pauker SG, Goldberg RJ, et al. Individualizing therapy to prevent long-term consequences of estrogen deficiency in postmenopausal women. Arch Intern Med 1999; 159: 1458–66
Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitive assessment of the epidemiologic evidence. Prev Med 1991; 20: 47–63
Anonymous. HRT has no effect on atherosclerosis. Scrip 2000; 2525: 21
McDonald CC, Stewart HJ. Fatal myocardial infarction in Scottish adjuvant tamoxifen trial. The Scottish Breast Cancer Committee. BMJ 1991; 303: 435–7
Rutqvist LE, Mattsson A. Cardiac and thromboembolic morbidity among postmenopausal women with early-stage breast cancer in a randomized trial of adjuvant tamoxifen. The Stockholm Breast Cancer Study Group. J Natl Cancer Inst 1993; 85: 1398–1406
McDonald CC, Alexander FE, Whyte BW, et al. Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a randomised trial. BMJ 1995; 311: 977–80
Costantino JP, Kuller LH, Ives DG, et al. Coronary heart disease mortality and adjuvant tamoxifen therapy. J Natl Cancer Inst 1997; 89: 776–82
Pilote L, Hlatky MA. Attitudes of women towards hormone therapy and prevention of heart disease. Am Heart J 1995; 129: 1237–8
Perez Gutthann S, Garcia Rodriguez LA, Castellsague J, et al. Hormone replacement therapy and risk of venous thromboembolism: population-based case-control study. BMJ 1997; 314: 796–800
Grodstein F, Stampfer MJ, Goldhaber SZ, et al. Prospective study of exogenous hormones and risk of pulmonary embolism in women. Lancet 1996; 348: 983–7
Jick H, Derby LE, Wald Meyers M, et al. Risk of hospital admission for idiopathic venous thromboembolism among users of postmenopausal estrogens. Lancet 1996; 348: 981–3
Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996; 348: 977–80
Kedar RP, Bourne TH, Powles PH, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. Lancet 1994; 343: 1318–21
Cecchini A, Ciatto S, Bonardi R, et al. Risk of endometrial cancer in breast cancer patients under long-term adjuvant treatment with tamoxifen. Tumori 1998; 84: 21–3
Jeal W, Barradell LB, McTavish D. Alendronate. A review of its pharmacological properties and therapeutic efficacy in postmenopausal osteoporosis. Drugs 1997; 53: 415–34
Kanis JA, Delmas P, Burckhardt P, et al. Guidelines for diagnosis and management of osteoporosis. Osteoporos Int 1997; 7: 390–406
Plosker GL, McTavish D. Intranasal salcatonin (salmon calcitonin): a review of its pharmacological properties and role in the management of postmenopausal osteoporosis. Drugs Aging 1996; 8: 378–400
Ashworth LE. Focus on raloxifene: a selective estrogen receptor modulator for prevention of osteoporosis in postmenopausal women. Formulary 1998; 33: 305–17
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clemett, D., Spencer, C.M. Raloxifene. Drugs 60, 379–411 (2000). https://doi.org/10.2165/00003495-200060020-00013
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200060020-00013