Abstract
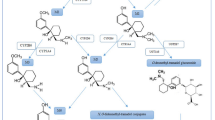
When peripheral decarboxylation is blocked by carbidopa or benserazide, the main metabolic pathway of levodopa is O-methylation by catechol-O-methyltransferase (COMT). Entacapone and tolcapone are new potent, selective and reversible nitrocatechol-type COMT inhibitors. Animal studies have demonstrated that entacapone mainly has a peripheral effect whereas tolcapone also inhibits O-methylation in the brain. In human volunteers, both entacapone and tolcapone dose-dependently inhibit the COMT activity in erythrocytes, improve the bioavailability and decrease the elimination of levodopa, and inhibit the formation of 3-O-methyldopa (3-OMD). Entacapone is administered with every scheduled dose of levodopa whereas tolcapone is administered 3 times daily. The different administration regimens for these agents are based on their different pharmacokinetic and pharmacodynamic profiles.
Both entacapone and tolcapone enhance and extend the therapeutic effect of levodopa in patients with advanced and fluctuating Parkinson’s disease. They prolong the duration of levodopa effect. Clinical studies show that they increase the daily ON time by an average 1 to 3 hours, improve the activities of daily living and allow daily levodopa dosage to be decreased. Correspondingly, they significantly reduce the daily OFF time. No comparative studies between entacapone and tolcapone have been performed. Tolcapone also appears to have a beneficial effect in patients with nonfluctuating Parkinson’s disease.
The main adverse effects of the COMT inhibitors are related to their dopaminergic and gastrointestinal effects. Enhancement of dopaminergic activity may cause an initial worsening of levodopa-induced adverse effects, such as dyskinesia, nausea, vomiting, orthostatic hypotension, sleep disorders and hallucinations. Levodopa dose adjustment is recommended to avoid these events. Tolcapone is associated with diarrhoea in about 16 to 18% of patients and entacapone in less than 10% of patients. Diarrhoea has led to discontinuation in 5 to 6% of patients treated with tolcapone and in 2.5% of those treated with entacapone. Urine discoloration to dark yellow or orange is related to the colour of COMT inhibitors and their metabolites. Elevated liver transaminase levels are reported in 1 to 3% of patients treated with tolcapone but very rarely, if at all, in patients treated with entacapone. The descriptions of acute, fatal fulminant hepatitis and potentially fatal neurological reactions, such as neuroleptic malignant syndrome and rhabdomyolysis, in association with tolcapone led to the suspension of its marketing authorisation in the European Community and Canada. In many other countries, the use of tolcapone is restricted to patients who are not responding satisfactorily to other therapies. Regular monitoring of liver enzymes is required if tolcapone is used. No such adverse reactions have so far been described for entacapone and no laboratory monitoring has been proposed.
COMT inhibitors added to levodopa therapy are beneficial, particularly in patients with fluctuating disease. They may be combined with other antiparkinsonian drugs, such as dopamine agonists, selegiline and anticholinergics without adverse interactions. They provide a new treatment possibility in patients with Parkinson’s disease who have problems with their present levodopa therapy.










Similar content being viewed by others
References
Axelrod J. O-methylation of epinephrine and other catechols in vitro and in vivo. Science 1957; 126: 400–1
Guldberg HC, Marsden CA. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev 1975; 27: 135–206
Ericsson AD. Potentiation of the L-dopa effect in man by the use of catechol-O-methyltransferase inhibitors. J Neurol Sci 1971; 14: 193–7
Reches A, Fahn S. Catechol-O-methyltransferase and Parkinson’s disease. Adv Neurol 1984; 40: 171–9
Männistö PT, Kaakkola S. Rationale for selective COMT inhibitors as adjuncts in the drug treatment of Parkinson’s disease. Pharmacol Toxicol 1990; 66: 317–23
Männistö PT, Ulmanen I, Taskinen J, et al. Catechol-O-methyltransferase (COMT) and COMT inhibitors. In: Sandier M, Smith J, editors. Design of enzyme inhibitors as drugs. Oxford: Oxford University Press, 1993; 623–46
Kaakkola S, Gordin A, Männistö PT. General properties and clinical possibilities of new selective inhibitors of catechol O-methyltransferase. Gen Pharmacol 1994; 25: 813–24
Pentikäinen PJ, Vuorela A, Järvinen M, et al. Human pharmacokinetics of OR-462, a new catechol-O-methyltransferase inhibitor. Eur J Clin Pharmacol 1989; 36 Suppl.: Al 10
Kaakkola S, Gordin A, Järvinen M, et al. Effect of a novel catechol-O-methyltransferase inhibitor, nitecapone, on the metabolism of L-dopa in healthy volunteers. Clin Neuropharmacol 1990; 13: 436–47
Bieck PR, Nilsson E, Antonin KH. Effect of the new selective COMT inhibitor CGP 28014 A on the formation of 3-O-methyldopa (3OMD) in plasma of healthy subjects. J Neural Transm Suppl 1990; 32: 387–91
Bieck PR, Antonin KH, Farger G, et al. Clinical pharmacology of the new COMT inhibitor CGP 28, 014. Neurochem Res 1993; 18: 1163–7
Feuerstein C, Tanche M, Serre F, et al. Does O-methyl-dopa play a role in levodopa-induced dyskinesias? Acta Neurol Scand 1977; 56: 79–82
Rivera-Calimlim L, Tandon D, Anderson F, et al. The clinical picture and plasma levodopa metabolite profile of parkinsonian nonresponders. Treatment with levodopa and decarboxylase inhibitor. Arch Neurol 1977; 34: 228–32
Tohgi H, Abe T, Kikuchi T, et al. The significance of 3-O-methyldopa concentrations in the cerebrospinal fluid in the pathogenesis of wearing-off phenomenon in Parkinson’s disease. Neurosci Lett 1991; 132: 19–22
Wade LA, Katzman R. 3-O-Methyldopa uptake and inhibition of L-dopa at the blood-brain barrier. Life Sci 1975; 17: 131–6
McKenzie GM, White HL. Evidence for the methylation of apomorphine by catechol-O-methyl-transferase in vivo and in vitro. Biochem Pharmacol 1973; 22: 2329–36
Symes AL, Lal S, Sourkes TL. Effect of catechol-O-methyltransferase inhibitors on brain apomorphine concentrations and stereotyped behaviour in the rat. J Pharm Pharmacol 1975; 27: 947–9
Coudore F, Durif F, Duroux E, et al. Effect of tolcapone on plasma and striatal apomorphine disposition in rats. Neuroreport 1997; 8: 877–80
Kohli JD, Horn PT, Glock D, et al. Dihydrexidine: a new potent peripheral dopamine D1 receptor agonist. Eur J Pharmacol 1993; 235: 31–5
Keränen T, Gordin A, Karlsson M, et al. Inhibition of soluble catechol-O-methyltransferase and single-dose pharmacokinetics after oral and intravenous administration of entacapone. Eur J Clin Pharmacol 1994; 46: 151–7
Dingemanse J, Jorga KM, Schmitt M, et al. Integrated pharmacokinetics and pharmacodynamics of the novel catechol-O-methyltransferase inhibitor tolcapone during first administration to humans. Clin Pharmacol Ther 1995; 57: 508–17
Heikkinen H, Pentikäinen PJ, Saraheimo M, et al. Pharmacokinetics of entacapone, a new COMT-inhibitor, in man: a study using stable isotope technique. New Trends Clin Neuropharm 1994; 8: 301
Jorga KM, Fotteler B, Heizmann P, et al. Pharmacokinetics and pharmacodynamics after oral and intravenous administration of tolcapone, a novel adjunct to Parkinson’s disease therapy. Eur J Clin Pharmacol 1998; 54: 443–7
Wikberg T, Vuorela A, Ottoila P, et al. Identification of major metabolites of the catechol-O-methyltransferase inhibitor entacapone in rats and humans. Drug Metab Dispos 1993; 21: 81–92
F. Hoffman-La Roche Ltd. Product monograph Tasmar. Basel: F. Hoffman-La Roche Ltd, 1997: 1–60
Da Prada M, Borgulya J, Napolitano A, et al. Improved therapy of Parkinson’s disease with tolcapone, a central and peripheral COMT inhibitor with an S-adenosyl-L-methionine-sparing effect. Clin Neuropharmacol 1995; 17: S26–S37
Dingemanse J, Jorga K, Zürcher G, et al. Multiple-dose clinical pharmacology of the catechol-O-methyl-transferase inhibitor tolcapone in elderly subjects. Eur J Clin Pharmacol 1996; 50: 47–55
Jorga K, Fotteler B, Wiegand U. Tolcapone does not change the pharmacokinetics and pharmacodynamics of the CYP2C9 substrate tolbutamide. Mov Disord 1997; 12 Suppl. 1: 100
Gordin A, Huupponen R, Rouru J, et al. Pharmacokinetics of entacapone and catechol-O-methyltransferase (COMT) inhibition after frequent multiple dosing of entacapone and effect on levodopa metabolism. Eur J Neurol 1998; 5 Suppl. 3: S165–S6
Jorga KM, Sedek G, Fotteler B, et al. Optimizing levodopa pharmacokinetics with multiple tolcapone doses in the elderly. Clin Pharmacol Ther 1997; 62: 300–10
Dingemanse J, Jorga K, Zürcher G, et al. Pharmacokinetic-pharmacodynamic interaction between the COMT inhibitor tolcapone and single-dose levodopa. Br J Clin Pharmacol 1995; 40: 253–62
Jorga K, Fotteler B, van Brummelen P. Why should tolcapone be given at a lower dose to patients with liver cirrhosis? Clin Pharmacol Ther 1997; 61: 183
Gordin A, Pentikäinen PP, Mäkimartti M, et al. Pharmacokinetics of the COMT inhibitor entacapone in liver failure and the effect of entacapone on liver function. Neurology 1998; 50 Suppl. 4: A387
Comtess Summary of Product Characteristics. Espoo, Finland: Orion Corp., 1998
Schultz E, Nissinen E. Inhibition of rat liver and duodenum soluble catechol-O-methyltransferase by a tight-binding inhibitor OR-462. Biochem Pharmacol 1989; 38: 3953–6
Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995; 34: 4202–10
Borges N, Vieira-Coelho MA, Parada A, et al. Studies on the tight-binding nature of tolcapone inhibition of soluble and membrane-bound rat brain catechol-O-methyltransferase. J Pharmacol Exp Ther 1997; 282: 812–7
Keränen T, Gordin A, Harjola VP, et al. The effect of catechol-O-methyl transferase inhibition by entacapone on the pharmacokinetics and metabolism of levodopa in healthy volunteers. Clin Neuropharmacol 1993; 16: 145–56
Sêdek G, Jorga K, Schmitt M, et al. Effect of tolcapone on plasma levodopa concentrations after coadministration with levodopa/carbidopa to healthy volunteers. Clin Neuropharmacol 1997; 20: 531–41
Jorga K, Fotteler B, Schmitt M, et al. The effect of COMT inhibition by tolcapone on tolerability and pharmacokinetics of different levodopa/benserazide formulations. Eur Neurol 1997; 38: 59–67
Myllylä VV, Sotaniemi KA, Mäkimartti M, et al. Effect of entacapone as an adjunct to Sinemet and Madopar on the pharmacokinetics of levodopa in parkinsonian patients. Mov Disord 1997; 12 Suppl. 1: 103
Jorga K, Fotteler B, Sedek G, et al. The effect of tolcapone on levodopa pharmacokinetics is independent of levodopa/carbidopa formulation. J Neurol 1998; 245: 223–30
Ahtila S, Kaakkola S, Gordin A, et al. Effect of entacapone, a COMT inhibitor, on the pharmacokinetics and metabolism of levodopa after administration of controlled-release levodopacarbidopa in volunteers. Clin Neuropharmacol 1995; 18: 46–57
Ruottinen HM, Rinne UK. A double-blind pharmacokinetic and clinical dose-response study of entacapone as an adjuvant to levodopa therapy in advanced Parkinson’s disease. Clin Neuropharmacol 1996; 19: 283–96
Ruottinen HM, Rinne UK. Effect of one month’s treatment with peripherally acting catechol-O-methyltransferase inhibitor, entacapone, on pharmacokinetics and motor response to levodopa in advanced parkinsonian patients. Clin Neuropharmacol 1996; 19: 222–33
Myllylä VV, Sotaniemi KA, Illi A, et al. Effect of entacapone, a COMT inhibitor, on the pharmacokinetics of levodopa and on cardiovascular responses in patients with Parkinson’s disease. Eur J Clin Pharmacol 1993; 45: 419–23
Nutt JG, Woodward WR, Beckner RM, et al. Effect of peripheral catechol-O-methyltransferase inhibition on the pharmacokinetics and pharmacodynamics of levodopa in parkinsonian patients. Neurology 1994; 44: 913–9
Tohgi H, Abe T, Yamazaki K, et al. Effects of the catechol-O-methyltransferase inhibitor tolcapone in Parkinson’s disease: correlations between concentrations of dopaminergic substances in the plasma and cerebrospinal fluid and clinical improvement. Neurosci Lett 1995; 192: 165–8
Roberts JW, Cora-Locatelli G, Bravi D, et al. Catechol-O-methyltransferase inhibitor tolcapone prolongs levodopa/carbidopa action in parkinsonian patients. Neurology 1993; 43: 2685–8
Limousin P, Pollak P, Pfefen JP, et al. Acute administration of levodopa-benserazide and tolcapone, a COMT inhibitor, Parkinson’s disease. Clin Neuropharmacol 1995; 18: 258–65
Yamamoto M, Yokochi M, Kuno S, et al. Effects of tolcapone, a catechol-O-methyltransferase inhibitor, on motor symptoms and pharmacokinetics of levodopa in patients with Parkinson’s disease. J Neural Transm 1997; 104: 229–36
Kaakkola S, Teräväinen H, Ahtila S, et al. Entacapone in combination with standard or controlled-release levodopa/carbidopa: a clinical and pharmacokinetic study in patients with Parkinson’s disease. Eur J Neurol 1995; 2: 341–7
Kuruma I, Bartholini G, Tissot R, et al. The metabolism of L-3-O-methyldopa, a precursor of dopa in man. Clin Pharmacol Ther 1971; 12: 678–82
Ruottinen HM, Rinne UK. Entacapone prolongs levodopa response in a one month double blind study in parkinsonian patients with levodopa related fluctuations. J Neurol Neurosurg Psychiatry 1996; 60: 36–40
Sundberg S, Scheinin M, Illi A, et al. The effects of the COMT inhibitor entacapone on haemodynamics and peripheral catecholamine metabolism during exercise. Br J Clin Pharmacol 1993; 36: 451–6
Illi A, Sundberg S, Koulu M, et al. COMT inhibition by highdose entacapone does not affect hemodynamics but changes catecholamine metabolism in healthy volunteers at rest and during exercise. Int J Clin Pharmacol Ther 1994; 32: 582–8
Zürcher G, Dingemanse J, Da Prada M. Potent COMT inhibition by Ro 40-7592 in the periphery and in the brain. Preclinical and clinical findings. Adv Neurol 1993; 60: 641–7
Lyytinen J, Kaakkola S, Ahtila S, et al. Simultaneous MAO-B and COMT inhibition in L-dopa-treated patients with Parkinson’s disease. Mov Disord 1997; 12: 497–505
Oechsner M, Stürenburg HJ, Buhmann C, et al. Elevated serum levels of dihydroxyphenylacetic acid (DOPAC) and dopamine after catechol-O-methyltransferase (COMT) inhibition. Eur J Neurol 1998; 5 Suppl. 3: S169
Davis TL, Roznoski M, Burns RS. Acute effects of COMT inhibition on L-DOPA pharmacokinetics in patients treated with carbidopa and selegiline. Clin Neuropharmacol 1995; 18: 333–7
Tasmar Summary of Product Characteristics. Basle, Switzerland: F. Hoffman-La Roche Ltd., 1998
Firnau G, Sood S, Chirakal R, et al. Metabolites of 6-[18F]fluoro-L-dopa in human blood. J Nucl Med 1988; 29: 363–9
Firnau G, Sood S, Chirakal R, et al. Cerebral metabolism of 6-[18F]fluoro-L-3,4-dihydroxyphenylalanine in the primate. J Neurochem 1987; 48: 1077–82
Guttman M, Leger G, Cedarbaum JM. OR-611 inhibits 3-Omethyldopa formation in primates. Neurology 1991; 41: 213
Günther I, Psylla M, Reddy GN, et al. Positron emission tomography in drug evaluation: influence of three different catechol-O-methyltransferase inhibitors on metabolism of [NCA] 6-[18F]fluoro-L-dopa in rhesus monkey. Nucl Med Biol 1995; 22: 921–7
Doudet DJ, Chan GL, Holden JE, et al. Effects of catechol-O-methyltransferase inhibition on the rates of uptake and reversibility of 6-fluoro-L-dopa trapping in MPTP-induced parkinsonism in monkeys. Neuropharmacology 1997; 36: 363–71
Psylla M, Günther I, Antonini A, et al. Cerebral 6-[18F]fluoroL-DOPA uptake in rhesus monkey: pharmacological influence of aromatic amino acid decarboxylase (AAAD) and catechol-O-methyltransferase (COMT) inhibition. Brain Res 1997; 767: 45–54
Holden JE, Doudet D, Endres CJ, et al. Graphical analysis of 6-fluoro-L-dopa trapping: effect of inhibition of catechol-O-methyltransferase. J Nucl Med 1997; 38: 1568–74
Laihinen A, Rinne JO, Rinne UK, et al. [18F]-6-fluorodopa PET scanning in Parkinson’s disease after selective COMT inhibition with nitecapone (OR-462). Neurology 1992; 42: 199–203
Sawle GV, Burn DJ, Morrish PK, et al. The effect of entacapone (OR-611) on brain [18F]-6-L-fluorodopa metabolism: implications for levodopa therapy of Parkinson’s disease. Neurology 1994; 44: 1292–7
Ishikawa T, Dhawan V, Chaly T, et al. Fluorodopa positron emission tomography with an inhibitor of catechol-O-methyltransferase: effect of the plasma 3-O-methyldopa fraction on data analysis. J Cerebral Blood Flow Metab 1996; 16: 854–63
Ruottinen H, Rinne J, Ruotsalainen U, et al. Striatal [18F]fluorodopa utilization after COMT inhibition with entacapone studied with PET in advanced Parkinson’s disease. J Neural Transm Park Dis Dem Sect 1995; 10: 91–106
Ruottinen HM, Rinne JO, Oikonen VJ, et al. Striatal 6-[18F] fluorodopa accumulation after combined inhibition of peripheral catechol-O-methyltransferase and monoamine oxidase type B: differing response in relation to presynaptic dopaminergic dysfunction. Synapse 1997; 27: 336–46
Merello M, Lees AJ, Webster R, et al. Effect of entacapone, a peripherally acting catechol-O-methyltransferase inhibitor, on the motor response to acute treatment with levodopa in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1994; 57: 186–9
Davis TL, Roznoski M, Burns RS. Effects of tolcapone in Parkinson’s patients taking L-dihydroxyphenylalanine/carbidopa and selegiline. Mov Disord 1995; 10: 349–51
Parkinson Study Group. Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Ann Neurol 1997; 42: 747–55
Rinne UK, Larsen JP, Siden Å, et al. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Neurology 1998; 51: 1309–14
Adler CH, Singer C, O’Brien C, et al. Randomized, placebocontrolled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa-carbidopa. Arch Neurol 1998; 55: 1089–95
Kurth MC, Adler CH, Hilaire MS, et al. Tolcapone improves motor function and reduces levodopa requirement in patients with Parkinson’s disease experiencing motor fluctuations: a multicenter, double-blind, randomized, placebo-controlled trial. Tolcapone Fluctuator Study Group I. Neurology 1997; 48: 81–7
Myllylä W, Jackson M, Larsen JP, et al. Efficacy and safety of tolcapone in levodopa-treated Parkinson’s disease patients with ‘wearing-off’ phenomenon: a multicentre, double-blind, randomized, placebo-controlled study. Eur J Neurol 1997; 4: 333–41
Baas H, Beiske AG, Ghika J, et al. Catechol-O-methyl-transferase inhibition with tolcapone reduces the ‘wearing off’ phenomenon and levodopa requirements in fluctuating parkinsonian patients. J Neurol Neurosurg Psychiatry 1997; 63: 421–8
Rajput AH, Martin W, Sainthilaire MH, et al. Tolcapone improves motor function in parkinsonian patients with the ‘wearing-off’ phenomenon: a double-blind, placebo-controlled, multicenter trial. Neurology 1997; 49: 1066–71
Limousin P, Pollak P, Gervason-Tournier CL, et al. Ro 40-7592, a COMT inhibitor, plus levodopa in Parkinson’s disease. Lancet 1993; 341: 1605
Roberts JW, Cora-Locatelli G, Bravi D, et al. Catechol-O-methyltransferase (COMT) inhibitor Ro 40-7592 prolongs duration of action of levodopa/carbidopa in parkinsonian patients. Neurology 1993; 43 Suppl. 2: A332
Dupont E, Burgunder JM, Findley LJ, et al. Tolcapone added to levodopa in stable parkinsonian patients: a double-blind placebo-controlled study. Mov Disord 1997; 12: 928–34
Waters CH, Kurth M, Bailey P, et al. Tolcapone in stable Parkinson’s disease: efficacy and safety of long-term treatment. Neurology 1997; 49: 665–71
Agid Y, Destee A, Durif F, et al. Tolcapone, bromocriptine, and Parkinson’s disease. Lancet 1997; 350: 712–3
Harper J, Vieira B. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Lancet 1998; 352: 578
Henry C, Wilson JA. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Lancet 1998; 351: 1965–6
Hauser RA, Molho E, Shale H, et al. A pilot evaluation of the tolerability, safety, and efficacy of tolcapone alone and in combination with oral selegiline in untreated Parkinson’s disease patients. Mov Disord 1998; 13: 643–7
Assal F, Spahr L, Hadengue A, et al. Tolcapone and fulminant hepatitis. Lancet 1998; 352: 958
EMEA. Recommendation for the suspension of the marketing authorisation for Tasmar (tolcapone) [press release]. Vol. CPMP/2457/98. London, 1998
Tasmar Product Label. Nutley (NJ): Roche Laboratories Inc., 1998
Jorga KM, Larsen JP, Beiske A, et al. The effect of tolcapone on the pharmacokinetics of benserazide. Eur J Neurol 1999; 6: 211–19
Tedroff J, Hartvig P, Bjurling P, et al. Central action of benserazide after COMT inhibition demonstrated in vivo by PET. J Neural Transm Gen Sect 1991; 85: 11–7
Illi A, Sundberg S, Ojala-Karlsson P, et al. The effect of entacapone on the disposition and hemodynamic effects of intravenous isoproterenol and epinephrine. Clin Pharmacol Ther 1995; 58: 221–7
Lyytinen J, Kaakkola S, Teräväinen H, et al. Comparison between the effects of L-dopa + entacapone and L-dopa + placebo on exercise capacity, haemodynamics and autonomic function in patients with Parkinson’s disease. Mov Disord 1997; 12 Suppl. 1: 103
Sedek G, Jorga K, Yoo K, et al. Lack of interaction between ephedrine and combination of tolcapone and sinemet. eurology 1996; 46 Suppl. 2: 374
Illi A, Sundberg S, Ojala-Karlsson P, et al. Simultaneous inhibition of catechol-O-methyltransferase and monoamine oxidase A: effects on hemodynamics and catecholamine metabolism in healthy volunteers. Clin Pharmacol Ther 1996; 59: 450–7
Illi A, Sundberg S, Ojala-Karlsson P, et al. Simultaneous inhibition of catecholamine-O-methylation by entacapone and neuronal uptake by imipramine: lack of interactions. Eur J Clin Pharmacol 1996; 51: 273–6
Jorga K, Fotteler B, Sedek G, et al. Effect of the COMT inhibitor tolcapone on the hemodynamics effects and tolerability of the combination treatment with levodopa/carbidopa and desipramine in healthy volunteers. Neurology 1997; 48 Suppl.: A185
Campbell NRC, Hasinoff BB. Iron supplements: a common cause of drug interactions. Br J Clin Pharmacol 1991; 31: 251–5
Orama M, Tilus P, Taskinen J, et al. Iron(III)-chelating properties of the novel catechol O-methyltransferase inhibitor entacapone in aqueous solution. J Pharm Sci 1997; 86: 827–31
Nutt JG. Catechol-O-methyltransferase inhibitors for treatment of Parkinson’s disease. Lancet 1998; 351: 1221–2
Khromova I, Voronina T, Kraineva VA, et al. Effects of selective catechol-O-methyltransferase inhibitors on single-trial passive avoidance retention in male rats. Behav Brain Res 1997; 86: 49–57
Moreau JL, Borgulya J, Jenck F, et al. Tolcapone: a potential new antidepressant detected in a novel animal model of depression. Behav Pharmacol 1994; 5: 344–50
Da Prada M, Borgulya J, Napolitano A, et al. Improved therapy of Parkinson’s Disease with tolcapone, a central and peripheral COMT inhibitor with an S-adenosyl-L-methione-sparing effect. Clin Neuropharmacol 1994; 17 Suppl. 3: S26–37
Yassin MS, Cheng H, Ekblom J, et al. Inhibitors of catecholamine metabolizing enzymes cause changes in S-adenosylmethionine and S-adenosylhomocysteine in the rat brain. Neurochem Int 1998; 32: 53–9
Gasparini M, Fabrizio E, Bonifati V, et al. Cognitive improvement during tolcapone treatment in Parkinson’s disease. J Neural Transm 1997; 104: 887–94
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaakkola, S. Clinical Pharmacology, Therapeutic Use and Potential of COMT Inhibitors in Parkinson’s Disease. Drugs 59, 1233–1250 (2000). https://doi.org/10.2165/00003495-200059060-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200059060-00004