Abstract
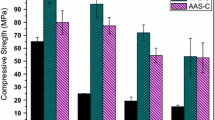
In the pursuit of new sources of aluminosilicates for alkaline cement manufacture, the present paper reports on the compositional and mineralogical assessment of the reactive potential of several types of bentonites. A number of factors affect strength development in these materials, in particular their potentially reactive silica and aluminium content. While bentonites normally have a high SiO2 content, the amount of reactive Al2O3 present is not always sufficient for the purpose at hand. The present study consequently also explored the effect of adding aluminium correctors (commercial sodium aluminate and bauxite) to the mix. The findings showed that de-hydroxylated bentonite reacted with an alkaline activator, yielding materials with cementitious properties. The addition of 10 % sodium aluminate raised mechanical strength, which was unaffected by the inclusion of bauxite. The primary reaction product was consistently found to be a (N,C)–A–S–H gel, with zeolites as the secondary products.







Similar content being viewed by others
References
Arjuman P, Silbee MR, Roy DM (1997) Quantitative determination of the crystalline and amorphous phases in low calcium fly ash. In: Justnes H (ed) Proceedings of the 10th international congress on the chemistry cement. Gothenburg, Sweden (3v 020, p 4)
Breck DW (1974) Zeolite molecular sieves. In: Robert E (ed) Structure, chemistry and use. Krieger Publishing Company, INC. Krieger Drive, Malabar, FL
Buchwald A, Hohmann M, Posern K, Brendler E (2009) The suitability of thermally activated illite/smectite clay as raw material for geopolymer binders. Appl Clay Sci 46:300–304
Chindaprasirt P, De Silva P, Sagoe-Crentsil K, Hanjitsuwan S (2012) Effect of SiO2 and Al2O3 on the settings and hardening of high calcium fly ash-based geopolymer systems. J Mat Sci 47:4876–4883
Criado M, Fernández-Jiménez A, de la Torre AG, Aranda MAG, Palomo A (2007) An XRD study of the effect of SiO2/Na2O ratio on the alkali activation of fly ash. Cem Concr Res 37:671–679
Criado M, Fernández-Jiménez A, Palomo A (2010) Alkali activation of fly ash. Part 3: effect of curing conditions on reaction and its graphical description. Fuel 89:3185–3192
Duxson P, Provis JL, Lukey GC, Mallicoat SW, Kriven WM, van Deventer JSJ (2005) Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf A 269:47–58
Duxson P, Mallicoat SW, Lukey GC, Kriven WM, van Deventer JSJ (2007) The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf A 292:8–20
Farmer VC (1975) The infrared spectra of minerals, mineralogical society, monograph 4. In: Farmer VC (ed). Mineralogical society, The Royal Society
Fernández-Jiménez A, Palomo A (2003) Characterization of fly ashes. Potential reactivity as alkaline cements. Fuel 82:2259–2265
Fernández-Jiménez A, Palomo A (2005) Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater 86:207–214
Fernández-Jiménez A, Palomo A, Sobrados I, Sanz J (2006) The role played by reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater 91:111–119
Fletcher RA, Mackenzie KJD, Nicholson CL, Shimada S (2005) The composition range of alumino silicate geopolymers. J Eur Cer Soc 25:1471–1477
Gadsden JA (1975) Infrared spectra of minerals and related inorganic compounds. Butterworths, London (England)
Garcia-Lodeiro I, Fernádez-Jiménez A, Pena P, Palomo A (2014) Alkali activation of synthetic aluminosilicate glasses. Ceram Int 40:5547–5558
García-Lodeiro I, Fernández-Jiménez A, Blanco MT, Palomo A (2008) FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J Sol–Gel Sci Technol 45:63–72
García-Lodeiro I, Fernández-Jiménez A, Palomo A (2013) Variation in hybrid cements over time. Alkaline activation of fly ash–portland cement blends. Cem Concr Res 52:112–122
Granizo ML, Alonso S, Blanco-Varela MT, Martinez-Ramirez S (2007) Alkali activation of metakaolins: parameters affecting mechanical, structural and microstructural properties. J Mater Sci 42:2934–2943
Hajimohammadi A, Provis JL, van Deventer JSJ (2010) Effect of alumina release rate on the mechanism of geopolymer gel formation. Chem Mater 22(5199–5208):5199
He C, Osback D, Makolicky E (1995) Pozzolanic Reactions of six principal clay minerals. Activation reactivity assessments and technological effects. Cem Concr Res 25(8):1691–1702
Hu M, Zhu X, Long F (2009) Alkali-activated fly ash-based geopolymers with zeolite or bentonite as additives. Cem Concr Compos 31:762–768
Huang WH (1997) Properties of cement fly -ash grout admixed with bentonite, silica fume or organic fiber. Cem Concr Res 27(3):395–406
Iler RK (1955) The chemistry of silica, ed. Wiley, New York
Kovalchuk G, Palomo A, Fernández-Jiménez A (2008) Activación alcalina de cenizas volantes. Relación entre el desarrollo mecánico resistente y la composición química de la ceniza. Materiales de la Construcción 58:35–52
Mozgawa W, Jastrzbski W, Handke M (2005) Vibrational spectra of D4R and D6R structural units. J Mol Struct 744–747(3)(SPEC. ISS.):663–670
Murray HH (2013) Applied clay mineralogy: occurrences, processing and applications of kaolins, bentonites, palygorskitesepiolite, and common clays, ed. Elsevier, USA
Palomo A, Grutzeck MW, Blanco MT (1999) Alkali activated fly ashes: a cement for the future. Cem Concr Res 29(8):1323–1329
Patterson H, Murray H (1972) Clays. Industrial minerals and rocks, ed. Elsevier, USA
Pimraksa K, Chindaprasirt P, Rungchet A, Sagoe-Crentsil K, Sato T (2011) Lightweight geopolymer made of highly porous siliceous materials with various Na2O/Al2O3 and SiO2/Al2O3 ratios. Mater Sci Eng A 528:6616–6623
Provis JL (2014) Geopolymers and other alkali activated materials: why, how, and what? Mater Struct 47:11–25
Ruiz-Santaquiteria C, Fernández-Jiménez A, Palomo A (2011) Quantitative determination of reactive SiO2 and Al2O3 in aluminosilicate materials. In: XIII international congress of cement chemistry. Madrid, Spain
Ruiz-Santaquiteria C, Fernádez-Jiménez A, Skibsted J, Palomo A (2013) Clay reactivity: production of alkali activated cements. Appl Clay Sci 73(1):11–16
Sagoe-Crentsil K, Brown T (2006) Some key materials and process parameters governing geopolymer binder performance. In: International conference on pozzolan, concrete and geopolymer khon kaen. Thailand, May 24–25, 2006
Seiffarth T, Hohmann M, Posern K, Kaps C (2013) Effect of thermal pre-treatment conditions of common clays on the performance of lay-based geopolymeric binders. Appl Clay Sci 73:35–41
Sharpe AG (1993) Química Inorgánica. Editorial Reverte S.A, Barcelona, Spain
Shi C, Fernández-Jimenez A, Palomo A (2011) New cements for the 21st century: the pursuit of an alternative to portland cement. Cem Concr Res 41:750–763
Silva PD, Sagoe-Crenstil K, Sirivivatnanon V (2007) Kinetics of geopolymerization: role of Al2O3 and SiO2. Cem Concr Res 37:512–518
Targan S, Olgun A, Erdogan Y, Sevinc V (2002) Effects of supplementary cementing materials on the properties of cement and concrete. Cem Concr Res 32:1551–1558
Targan S, Olgun A, Erdogan Y, Sevinc V (2003) Influence of natural pozzolan, colemanite ore waste, bottom ash, and fly ash on the properties of Portland cement. Cem Concr Res 33(8):1175–1182
Weaver CE, Pollard L (1973) The chemistry of clays minerals, ed. Elsevier, USA
Zeng Q, Li K (2104) Reaction and microstructure of cement–fly-ash system. Mater Struct. doi:10.1617/s11527-014-0266-y
Acknowledgments
This research was funded by the Spanish Ministry of Economy and Competitiveness under Project BIA 2010-17530 and supported by a Post-graduate Studies Council contract (JAE DOC 2011), co-funded by the Spanish National Research Council and the European Social Fund (FSE).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
García-Lodeiro, I., Cherfa, N., Zibouche, F. et al. The role of aluminium in alkali-activated bentonites. Mater Struct 48, 585–597 (2015). https://doi.org/10.1617/s11527-014-0447-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1617/s11527-014-0447-8