Abstract
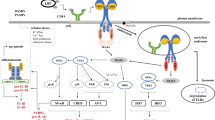
Fcγ receptors are among the best-studied phagocytic receptors. The key features of Fcγ receptor-mediated phagocytosis include phagocytic cup formation by extensive actin cytoskeletal rearrangements, particle engulfment, and the release of proinflammatory mediators such as cytokines and reactive oxygen species. These events are elegantly regulated by the simultaneous engagement of activating and inhibitory Fcγ receptors and by intracellular signaling molecules. Extensive studies in the past several years have defined the molecular mechanisms of the phagocytic process. The purpose of this review is to revisit some of the well-established signaling pathways as well as to summarize the new findings in this field.
Similar content being viewed by others
References
Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5:85–87.
Ravetch JV. Fc receptors. Curr Opin Immunol. 1997;9:121–125.
Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432.
Unkeless JC, Jin J. Inhibitory receptors, ITIM sequences and phosphatases. Curr Opin Immunol. 1997;9:338–343.
Cambier JC. Antigen and Fc receptor signaling: the awesome power of the immunoreceptor tyrosine-based activation motif (ITAM). J Immunol. 1995;155:3281–3285.
Isakov N. Immunoreceptor tyrosine-based activation motif (ITAM), a unique module linking antigen and Fc receptors to their signaling cascades. J Leukoc Biol. 1997;61:6–16.
Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560.
Nimmerjahn F, Ravetch JV. Fcγ receptors: old friends and new family members. Immunity. 2006;24:19–28.
Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001; 19:275–290.
Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR γ chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76: 519–529.
Sanchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. J Leukoc Biol. 1998;63:521–533.
Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcγRIV a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51.
Cooney DS, Phee H, Jacob A, Coggeshall KM. Signal transduction by human-restricted FcγRIIa involves three distinct cytoplasmic kinase families leading to phagocytosis. J Immunol. 2001;167: 844–854.
Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J Biol Chem. 1994;269:8878–8884.
Tridandapani S, Lyden TW, Smith JL, Carter JE, Coggeshall KM, Anderson CL. The adapter protein LAT enhances Fcγ receptormediated signal transduction in myeloid cells. J Biol Chem. 2000; 275:20480–20487.
Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637.
Greenberg S, Chang P, Silverstein SC Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534.
Salcedo TW, Kurosaki T, Kanakaraj P, Ravetch JV, Perussia B. Physical and functional association of p56lck with Fc gamma RIIIA (CD16) in natural killer cells. J Exp Med. 1993;177: 1475–1480.
Duchemin AM, Anderson CL. Association of non-receptor protein tyrosine kinases with the Fc gamma RI/gamma-chain complex in monocytic cells. J Immunol. 1997;158:865–871.
Crowley MT, Costello PS, Fitzer-Attas CJ, et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J Exp Med. 1997;186:1027–1039.
Agarwal A, Salem P, Robbins KC. Involvement of p72syk, a protein-tyrosine kinase, in Fc gamma receptor signaling. J Biol Chem. 1993;268:15900–15905.
Ghazizadeh S, Bolen JB, Fleit HB. Tyrosine phosphorylation and association of Syk with Fc gamma RII in monocytic THP-1 cells. Biochem J.1995;305(pt 2):669–674.
Kiefer F, Brumell J, Al Alawi N, et al. The Syk protein tyrosine kinase is essential for Fcγ receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–4220.
Matsuda M, Park JG, Wang DC, Hunter S, Chien P, Schreiber AD. Abrogation of the Fc gamma receptor IIA-mediated phagocytic signal by stem-loop Syk antisense oligonucleotides. Mol Biol Cell. 1996;7:1095–1106.
Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86: 4389–4399.
Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages.J Cell Biol. 1996;135:1249–1260.
Vanhaesebroeck B, Leevers SJ, Panayotou G,Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272.
Ninomiya N, Hazeki K, Fukui Y, et al. Involvement of phosphatidylinositol 3-kinase in Fc gamma receptor signaling. J Biol Chem. 1994;269:22732–22737.
Kanakaraj P, Duckworth B, Azzoni L, Kamoun M, Cantley LC, Perussia B. Phosphatidylinositol-3 kinase activation induced upon Fc gamma RIIIA-ligand interaction. J Exp Med. 1994;179:551–558.
Vossebeld PJ, Homburg CH, Schweizer RC, et al. Tyrosine phosphorylation-dependent activation of phosphatidylinositide 3- kinase occurs upstream of Ca2+-signalling induced by Fcgamma receptor cross-linking in human neutrophils. Biochem J. 1997; 323(pt 1):87–94.
Leverrier Y, Okkenhaug K, Sawyer C, Bilancio A, Vanhaesebroeck B, Ridley AJ. Class I phosphoinositide 3-kinase p110β is required for apoptotic cell and Fcγ receptor-mediated phagocytosis by macrophages. J Biol Chem. 2003;278:38437–38442.
Carpenter CL, Duckworth BC, Auger KR, Cohen B, Schaffhausen BS, Cantley LC Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990;265:19704–19711.
Carpenter CL, Auger KR, Chanudhuri M, et al. Phosphoinositide 3-kinase is activated by phosphopeptides that bind to the SH2 domains of the 85-kDa subunit. J Biol Chem. 1993;268:9478–9483.
Lowry MB, Duchemin AM, Coggeshall KM, Robinson JM, Anderson CL. Chimeric receptors composed of phosphoinositide 3-kinase domains and Fcγ receptor ligand-binding domains mediate phagocytosis in COS fibroblasts. J Biol Chem. 1998;273:24513–24520.
Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247.
Beemiller P, Hoppe AD, Swanson JA. A phosphatidylinositol-3- kinase-dependent signal transition regulates ARF1 and ARF6 during Fcγ receptor-mediated phagocytosis. PLoS Biol. 2006;4:e162.
D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358.
Allen LA, Allgood JA, Han X, Wittine LM. Phosphoinositide3- kinase regulates actin polymerization during delayed phagocytosis of Helicobacter pylori. J Leukoc Biol. 2005;78:220–230.
Ganesan LP, Wei G, Pengal RA, et al. The serine/threonine kinase Akt promotes Fcγ receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J Biol Chem. 2004;279:54416–54425.
Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179.
Ueyama T, Eto M, Kami K, et al. Isoform-specific membrane targeting mechanism of Rac during FcγR-mediated phagocytosis: positive charge-dependent and independent targeting mechanism of Rac to the phagosome. J Immunol. 2005;175:2381–2390.
Nishihara H, Maeda M, Oda A, et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100: 3968–3974.
ten Klooster JP, Jaffer ZM, Chernoff J, Hordijk PL. Targeting and activation of Rac1 are mediated by the exchange factor β-Pix. J Cell Biol. 2006;172:759–769.
Liu BP, Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not β1 integrins. Mol Cell Biol. 2000;20:7160–7169.
Miranti CK, Leng L, Maschberger P, Brugge JS, Shattil SJ. Identification of a novel integrin signaling pathway involving the kinase Syk and the guanine nucleotide exchange factor Vav1. Curr Biol. 1998;8:1289–1299.
Hall AB, Gakidis MA, Glogauer M et al. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcγR- and complement-mediated phagocytosis. Immunity. 2006;24: 305–316.
Zhang J, Guo J, Dzhagalov I, He YW. An essential function for the calcium-promoted Ras inactivator in Fcγ receptor-mediated phagocytosis. Nat Immunol. 2005;6:911–919.
Dorseuil O, Reibel L, Bokoch GM, Camonis J, Gacon G. The Rac target NADPH oxidase p67phox interacts preferentially with Rac2 rather than Rac1. J Biol Chem. 1996;271:83–88.
Kim C, Dinauer MC. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol. 2001;166: 1223–1232.
Yamauchi A, Kim C, Li S, et al. Rac2-deficient murine macrophages have selective defects in superoxide production and phagocytosis of opsonized particles. J Immunol. 2004;173:5971–5979.
Hoppe AD, Swanson JA. Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis. Mol Biol Cell. 2004;15: 3509–3519.
Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048.
Kiener PA, Rankin BM, Burkhardt AL, et al. Cross-linking of Fc gamma receptor I (Fc gamma RI) and receptor II (Fc gamma RII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993;268:24442–24448.
Upshaw JL, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. The isoforms of phospholipase C-γ are differentially used by distinct human NK activating receptors. J Immunol. 2005;175:213–218.
Dusi S, Donini M, Della Bianca V, Rossi F. Tyrosine phosphorylation of phospholipase C-γ2 is involved in the activation of phosphoinositide hydrolysis by Fc receptors in human neutrophils. Biochem Biophys Res Commun. 1994;201:1100–1108.
Botelho RJ, Teruel M, Dierckman R, et al. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol. 2000;151:1353–1368.
Virgilio DiF, Meyer BC, Greenberg S, Silverstein SC. Fc receptormediated phagocytosis occurs in macrophages at exceedingly low cytosolic Ca2+ levels. J Cell Biol. 1988;106:657–666.
McNeil PL, Swanson JA, Wright SD, Silverstein SC, Taylor DL. Fc-receptor-mediated phagocytosis occurs in macrophages without an increase in average [Ca++]i. J Cell Biol. 1986;102:1586–1592.
Mandeville JT, Maxfield FR. Calcium and signal transduction in granulocytes. Curr Opin Hematol. 1996;3:63–70.
Larsen EC, DiGennaro JA, Saito N, et al. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J Immunol. 2000;165:2809–2817.
Zheleznyak A, Brown EJ. Immunoglobulin-mediated phagocytosis by human monocytes requires protein kinase C activation: evidence for protein kinase C translocation to phagosomes. J Biol Chem. 1992;267:12042–12048.
Larsen EC, Ueyama T, Brannock PM, et al. A role for PKC-ε in FcγR-mediated phagocytosis by RAW 264.7 cells. J Cell Biol. 2002; 159:939–944.
Muid RE, Dale MM, Davis PD, et al. A novel conformationally restricted protein kinase C inhibitor, Ro 31-8425, inhibits human neutrophil superoxide generation by soluble, particulate and post- receptor stimuli. FEBS Lett. 1991;293:169–172.
Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med. 1999;189:179–185.
Park JB. Phagocytosis induces superoxide formation and apoptosis in macrophages. Exp Mol Med. 2003;35:325–335.
Hunter S, Indik ZK, Kim MK, Cauley MD, Park JG, Schreiber AD. Inhibition of Fcγ receptor-mediated phagocytosis by a nonphago- cytic Fcγ receptor. Blood. 1998;91:1762–1768.
Joshi T, Ganesan LP, Cao X, Tridandapani S. Molecular analysis of expression and function of hFcγRIIbl and b2 isoforms in myeloid cells. Mol Immunol. 2006;43:839–850.
Liu Y, Masuda E, Blank MC, et al. Cytokine-mediated regulation of activating and inhibitory Fcγ receptors in human monocytes. J Leukoc Biol. 2005;77:767–776.
Pricop L, Redecha P, Teillaud JL, et al. Differential modulation of stimulatory and inhibitory Fcγ receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001;166:531–537.
Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of FcγRIIb in human monocytic cells. J Biol Chem. 2002;277: 5082–5089.
Chacko GW, Tridandapani S, Damen JE, Liu L, Krystal G, Coggeshall KM. Negative signaling in B lymphocytes induces tyrosine phosphorylation of the 145-kDa inositol polyphosphate 5- phosphatase, SHIP. J Immunol. 1996;157:2234–2238.
Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature. 1996;383:263–266.
Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301.
Rohrschneider LR, Fuller JF, Wolf I, Liu Y, Lucas DM. Structure, function, and biology of SHIP proteins. Genes Dev. 2000;14: 505–520.
Aman MJ, Lamkin TD, Okada H, Kurosaki T, Ravichandran KS. The inositol phosphatase SHIP inhibits Akt/PKB activation in B cells. J Biol Chem. 1998;273:33922–33928.
Scharenberg AM, El Hillal O, Fruman DA, et al. Phosphatidylinositol- 3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972.
Tridandapani S, Kelley T, Pradhan M, Cooney D, Justement LB, Coggeshall KM. Recruitment and phosphorylation of SH2-containing inositol phosphatase and Shc to the B-cell Fc gamma immunorecep- tor tyrosine-based inhibition motif peptide motif. Mol Cell Biol. 1997; 17:4305–4311.
Tridandapani S, Pradhan M, LaDine JR, Garber S, Anderson CL, Coggeshall KM. Protein interactions of Src homology 2 (SH2) domain-containing inositol phosphatase (SHIP): association with Shc displaces SHIP from FcγRIIb in B cells. J Immunol. 1999;162: 1408–1414.
Cox D, Dale BM,Kashiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5′- phosphatase (SHIP) in phagocytosis mediated by Fcγ receptors and complement receptor 3 (αMβ2; CD11b/CD18). J Exp Med. 2001;193:61–71.
Nakamura K, Malykhin A, Coggeshall KM. The Src homology 2 domain-containing inositol 5-phosphatase negatively regulates Fcβ receptor-mediated phagocytosis through immunoreceptor tyrosine- based activation motif-bearing phagocytic receptors. Blood. 2002; 100:3374–3382.
Tridandapani S, Wang Y, Marsh CB, Anderson CL. Src homology 2 domain-containing inositol polyphosphate phosphatase regulates NF-KB-mediated gene transcription by phagocytic FcγRs in human myeloid cells. J Immunol. 2002;169:4370–4378.
Ganesan LP, Joshi T, Fang H, et al. FcγR-induced production of superoxide and inflammatory cytokines is differentially regulated by SHIP through its influence on PI3K and/or Ras/Erk pathways. Blood. 2006;108:718–725.
Pengal RA, Ganesan LP, Fang H, Marsh CB, Anderson CL, Tridandapani S. SHIP-2 inositol phosphatase is inducibly expressed in human monocytes and serves to regulate Fcγ receptor-mediated signaling. J Biol Chem. 2003;278:22657–22663.
Ai J, Maturu A, Johnson W, Wang Y, Marsh CB, Tridandapani S.The inositol phosphatase SHIP-2 down-regulates FcγR-mediated phagocytosis in murine macrophages independently of SHIP-1. Blood. 2006;107:813–820.
Sulis ML, Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–483.
Kim JS, Peng X, De PK, Geahlen RL, Durden DL. PTEN controls immunoreceptor (immunoreceptor tyrosine-based activation motif) signaling and the activation of Rac. Blood. 2002;99: 694–697.
Cao X, Wei G, Fang H, et al. The inositol 3-phosphatase PTEN negatively regulates Fcγ receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J Immunol. 2004;172:4851–4857.
Kant AM, De P, Peng X, et al. SHP-1 regulates Fcγ receptor-mediated phagocytosis and the activation of RAC. Blood. 2002;100: 1852–1859.
Ganesan LP, Fang H, Marsh CB, Tridandapani S. The protein-tyrosine phosphatase SHP-1 associates with the phosphorylated immunoreceptor tyrosine-based activation motif of FcγRIIa to modulate signaling events in myeloid cells. J Biol Chem. 2003;278: 35710–35717.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Joshi, T., Butchar, J.P. & Tridandapani, S. Fcγ Receptor Signaling in Phagocytes. Int J Hematol 84, 210–216 (2006). https://doi.org/10.1532/IJH97.06140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1532/IJH97.06140