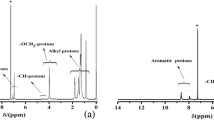
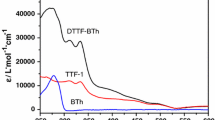
Abstract
Diazene sulphonates are readily available compounds which are soluble in water and polar solvents. They strongly absorb the UV-VIS light and they decompose under UV irradiation via radical or ionic intermediates. These properties render them valuable, e.g. for analytical purposes and for photo-printing. In this presentation, it will be demonstrated that polymers with a pendant diazene sulphonate function as cross-linking agents and with pendant aromatic amines as hole-transporters can be considered as useful materials for organic light-emitting device (OLED) technology.
Similar content being viewed by others
References
Bamberger, E. (1894). Ueber Stereomerie bei Diazoverbindungen und die Natur der Isodiazokorper. Berichte der deutschen chemischen Gesellschaft, 27, 2582–2595. DOI: 10.1002/cber.189402702264. (in German)
Dauth, J. (1991). Neue Azo-, Triazen- und Peroxyverbindungen im Thermo- und Photodruck. Ph.D. thesis, University of Bayreuth, Germany. (in German)
Dauth, J., Nuyken, O., Strohriegl, P., & Voit, B. (1992). Watersoluble photoresins based on azosulfonates. Die Makromolekulare Chemie, 193, 723–734. DOI: 10.1002/macp.1992. 021930318.
de Jonge, J., & Dijkstra, R. (1956). Some photochemical properties of alkali salts of aryldiazosulphonic acids. Recueil des Travaux Chimiques des Pays-Bas, 75, 290–300. DOI: 10.1002/recl. 19560750308.
Dunkin, I. R., Gittinger, A., Sherrington, D. C., & Whittaker, P. (1996). Synthesis, characterization and applications of azo-containing photodestructible surfactants. Journal of the Chemical Society, Perkin Transactions, 2, 1996, 1837–1842. DOI: 10.1039/p29960001837.
Eastoe, J. (2006). Photo-destructible surfactants in microemulsions. In W. Richtering (Ed.), Smart colloidal materials (Series: Progress in colloid and polymer science, Vol. 133, pp. 106–110). Berlin, Germany: Springer. DOI: 10.1007/3-54032702-9-17.
Faber, R., Mielke, G. F., Rapta, P., Stasko, A., & Nuyken, O. (2000). Anodic oxidation of novel hole-transporting materials derived from tetraarylbenzidines. Electrochemical and spectroscopic characterization. Collection of Czechoslovak Chemical Communications, 65, 1403–1418. DOI: 10.1135/cccc20001403.
Faber, R. (2001). Neue Polymere fur organische LEDs auf der Basis von Fluorenylidenverknüpften Oligo(p-phenylen)en. Ph.D. thesis, Technical University of Munich, Germany. (in German)
Franzke, D., Voit, B., Nuyken, O., & Wokaun, A. (1992a). Wavelength-dependent photolysis of 3-vinyl-phenyl-azosul-fonate. Journal of Photochemistry and Photobiology A: Chemistry, 68, 205–212. DOI: 10.1016/1010-6030(92)85184-v.
Franzke, D., Voit, B., Nuyken, O., & Wokaun, A. (1992b). Pulsed ultraviolet laser photolysis of substituted phenyl azo-sulfonates: Wavelength dependent effects. Molecular Physics, 77, 397–409. DOI: 10.1080/00268979200102511.
Freeman, H. C., & Le Fevre, R. J. W. (1951). Hantzsch’s isomeric diazosulphonates. Journal of the Chemical Society, 1951, 415–428. DOI: 10.1039/jr9510000415.
Geckeler, K. E., Nuyken, O., Schnoller, U., Thünemann, A., & Voit, B. (2000). Complexing behavior of diazosulfonate polymer. In Y. Yagci, M. K. Mishra, O. Nuyken, K. Ito, & K. Wnek (Eds.), Tailored polymers & applications (pp. 287–296). Leiden, the Netherlands: VSP International Publishers.
Georgi, U. (2014). Synthese photoaktiver Polymere zur optischen Strukturierung dunner Schichten. Ph.D. thesis, Dresden University of Technology, Germany. (in German)
Girad, E. (1972). FR Patent No. 2090801. Paris, France: INPI.
Hantzsch, A. (1894). Ueber Stereoisomerie bei Diazoverbindungen und die Natur der Isodiazokoürper. Berichte der deutschen chemischen Gesellschaft, 27, 1702–1725. DOI: 10.1002/cber.189402702110. (in German)
Hantzsch, A., & Borghaus, H. (1897). Ueber Bis-Diazoniumsalze. Berichte der deutschen chemischen Gesellschaft, 30, 9293. DOI: 10.1002/cber.18970300115. (in German)
Hodgson, H. H., & Marsden, E. (1943). The decomposition of diazo-compounds in neutral solution. Some observations on the diazo-coupling reaction. Journal of the Chemical Society, 1943, 379–380. DOI: 10.1039/jr9430000379.
Jonker, H., Thijssens, T. P. G. W., & van Beek, L. K. H. (1968). Properties of diazosulfonates. Part VI: Quantum yields for the photolysis of 2-methoxybenzenediazonium and for the photo-isomerization of 2-methoxybenzene-trans-diazosulfonate. Recueil des Travaux Chimiques des Pays-Bas, 87, 997–1005. DOI: 10.1002/recl.19680870903.
Kerber, R., Nuyken, O., & Dorn, M. (1978). “Über die Copolymerization von (3-vinylphenylazo)-methylmalonodinitril mit Styrol 7. Mitteilung uüber Azoinitiatoren. Die Makromolekulare Chemie, 179, 1803–1814. DOI: 10.1002/macp.1978.0217 90715.
Knepper, T. (1987). Synthese unsymmetrischer Azoverbindungen und ihr Verhalten bei Photoreaktionen. Diploma thesis, University of Mainz, Germany. (in German)
Lewis, E. S., & Suhr, H. (1959). Untersuchungen über die Reaktion von Diazoniumsalzen mit Sulfit. Chemische Berichte, 92, 3031–3043. DOI: 10.1002/cber.19590921205. (in German)
Lichnerova, E. (2005). Neue vernetzbare Polymere fur die OLED-Technologie. Ph.D. thesis, Technical University of Munich, Germany. (in German)
Liu, S., Jiang, X., Ma, H., Liu, M. S., & Jen, A. K. Y. (2000). Triarylamine-containing poly(perfluorocyclobutane) as hole-transporting material for polymer light-emitting diodes. Macromolecules, 33, 3514–3517. DOI: 10.1021/ma0002038.
Ma, B., Lauterwasser, F., Deng, L., Zonte, C. S., Kim, B. J., Fréchet, J. M. J., Borek, C., & Thompson, M. E. (2007). New thermally cross-linkable polymer and its application as a hole-transporting layer for solution processed multilayer organic light emitting diodes. Chemistry of Materials, 19, 4827–4832. DOI: 10.1021/cm0715500.
Matusche, P. (1995). Polymere Diazosulfonate: Synthese, Eigenschaften und Anwendungen im Photodruck sowie als Nachweisreagenz fur phenolische Naturstoffe. Ph.D. thesis, Technical University of Munich, Germany. (in German)
Matusche, P., Nuyken, O., Voit, B., Van Damme, M., Vermeersch, J., De Winter, W., & Alaerts, L. (1995). Water soluble photoresins based on polymeric azo compounds. Reactive Polymers, 24, 271–278. DOI: 10.1016/0923-1137(94)00093-k.
Matusche, P., Nuyken, O., & Voit, B. (1997a). Low-molar mass and polymeric diazosulfonates as disguised diazonium ions for application as analytical reagents in quick assays. Die Angewandte Makromolekulare Chemie, 250, 45–65. DOI: 10.1002/apmc.1997.052500104.
Matusche, P., Nuyken, O., Voit, B., & Van Damme, M. (1997b). Water soluble and photoactive copolymers containing amidic aryldiazosulfonate groups. Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 34, 201–209. DOI: 10.1080/10601329708014947.
Mezger, T., Nuyken, O., Meindl, K., & Wokaun, A. (1996). Light decomposable emulsifiers: application of alkyl-substituted aromatic azosulfonates in emulsion polymerization. Progress in Organic Coatings, 29, 147–157. DOI: 10.1016/s0300-9440(96)00666-2.
Neta, P., & Huie, R. E. (1985). Freeradical chemistry of sulfite. Environmental Health Perspectives, 64, 209–217.
Nuyken, O., & Voit, B. (1989). Water-soluble and photosensitive copolymers from methyl methacrylate and sodium — vinylphenylazosulfonate. Die Makromolekulare Chemie, 190, 1325–1332. DOI: 10.1002/macp.1989.021900613.
Nuyken, O., Knepper, T., Voit, B., & Pask, S. D. (1991). U.S. Patent No. 5037961 (A). Washington, D.C., USA: U.S. Patent and Trademark Office.
Nuyken, O., Dauth, J. W., & Vermeersch, J. T. (1992). European Patent Application No. 0507008 (A1). Munich, Germany: European Patent Office.
Nuyken, O., Dauth, J., Voit, B., & Strohriegl, P. (1993). Hightech applications of polymeric azo compounds. In J. Kahovec (Ed.), Macromolecules 1992 Invited lectures of the 34th IU-PAC International Symposium on Macromolecules (pp. 119–131). Utrecht, the Netherlands: VSP.
Nuyken, O., Meindl, K., Wokaun, A., & Mezger, T. (1994). Photolabile surfactants based on the diazosulfonate group I. 4-n-Alkylbenzenediazosulfonates. Journal of Photochemistry and Photobiology A: Chemistry, 81, 45–53. DOI: 10.1016/1010-6030(93)03771-8.
Nuyken, O., Meindl, K., Wokaun, A., & Mezger, T. (1995). Photolabile surfactants based on the diazosulphonate group 2. 4-(Acyloxy)benzenediazosulphonates and 4-(acylamino)benz-enediazosulphonates. Journal of Photochemistry and Photobiology A: Chemistry, 85, 291–298. DOI: 10.1016/1010-6030(94)03919-1.
Nuyken, O., & Voit, B. (1997). The photoactive diazosulfonate group and its role in polymer chemistry. Macromolecular Chemistry and Physics, 198, 2337–2372. DOI: 10.1002/macp.1997.021980801.
Nuyken, O., Jungermann, S., Wiederhirn, V., Bacher, E., & Meerholz, K. (2006). Modern trends in organic light-emitting devices (OLEDs). Monatshefte für Chemie, 137, 811–824. DOI: 10.1007/s00706-006-0490-4.
Nuyken, O. (2014). Trends in den Polymerwissenschaften. Chemie in Unserer Zeit, 48, 270–283. DOI: 10.1002/ciuz.201 400667. (in German)
Rapta, P., Stasko, A., Bustin, D., Nuyken, O., & Voit, B. (1992). Electrochemical reduction of azo sulfonates and sulfones. A cyclic voltammetry and EPR study. Journal of the Chemical Society, Perkin Transactions, 2, 1992, 2049–2055. DOI: 10.1039/p29920002049.
Reichenbach, P. (2011). Metallische Nanoantennen: Frequenzverdopplung und photochemische Reaktionen auf kleinen Skalen. Ph.D. thesis, Dresden University of Technology, Germany. (in German)
Schelter, J., Mielke, G. F., Kohnen, A., Wies, J., Kober, S., Nuyken, O., & Meerholz, K. (2010). Novel non-conjugated main-chain hole-transporting polymers for organic electronics application. Macromolecular Rapid Communications, 31, 1560–1567. DOI: 10.1002/marc.201000125.
Schmitt, K., & Glutz, L. (1869). Ueber Diazophenole. Berichte der deutschen chemischen Gesellschaft, 2, 51–53. DOI: 10.1002/cber.18690020124. (in German)
Schnoller, U. (1996). Synthese und Eigenschaften neuer diazo-sulfonathaltiger Polymere fur die Anwendung im Thermo- und Photodruck und in Polyelektrolyt-Tensid-Komplexen. Ph.D. thesis, Technical University of Munich, Germany. (in German)
Spiertz, E., & van Beek, L. (1974). U.S. Patent No. 3802886 (A). Washington, D.C., USA: U.S. Patent and Trademark Office.
Stampor, W., & Mróz, W. (2007). Electroabsorption in triphe-nylamine-based hole-transporting materials for organic light-emitting diodes. Chemical Physics, 331, 261–269. DOI: 10.1016/j.chemphys.2006.10.014.
Staško, A., Szaboova, K., Cholvad, V., Nuyken, O., & Dauth, J. (1993). The photochemical decomposition of azo compounds (a spin trap study). Journal of Photochemistry and Photobiology A: Chemistry, 69, 295–304. DOI: 10.1016/1010-6030(93)85095-p.
Thünemann, A. F., Schnöller, U., Nuyken, O., & Voit, B. (1999). Self-assembled complexes of diazosulfonate polymers with low surface energies. Macromolecules, 32, 7414–7421. DOI: 10.1021/ma990868d.
Trogisch, S. (2003). Optische Strukturierung ultradünner funktioneller Polymerfilme. Ph.D. thesis, Dresden University of Technology, Germany. (in German)
van Aert, H., van Damme, M., Nuyken, O., Schnoller, U., Eichhorn, K. J., Grundke, K., & Voit, B. (2001). Imagewise structuring of diazosulfonate polymer films by UV light and laser irradiation — a comparison. Macromolecular Materials and Engineering, 286, 488–496. DOI: 10.1002/1439-2054(200108)286:8< 488::AID-MAME488> 3.0.CO;2-F.
van Beek, L. K. H., Hellferich, J., Jonker, H., & Thijssens, T. P. G. W. (1967a). Properties of diazosulfonates. Part I: The dissociation of methoxybenzenediazosulfonates. Recueil des Travaux Chimiques des Pays-Bas, 86, 405–409. DOI: 10.1002/recl.19670860409.
van Beek, L. K. H., Hellferich, J., Jonker, H., & Thijssens, T. P. G. W. (1967b). Properties of diazosulfonates. Part II: The rate of the reaction between 2-methoxybenzenediazonium and sulfite ions. Recueil des Travaux Chimiques des Pays-Bas, 86, 749–754. DOI: 10.1002/recl.19670860710.
van der Veen, J., Helfferich, J., & van Beek, L. K. H. (1966). Photo isomerization of methoxybenzene diazosulfonates. Recueil des Travaux Chimiques des Pays-Bas, 85, 895–898. DOI: 10.1002/recl.19660850907.
Voit, B., Braun, F., Gernert, M., Sieczkowska, B., Millaruelo, M., Messerschmidt, M., Mertig, M., & Opitz, J. (2006). Photolabile and thermally labile polymers as templates and for surface patterning. Polymers for Advanced Technologies, 17, 691–693. DOI: 10.1002/pat.793.
Voit, B., Braun, F., Stadermann, J., & Riedel, M. (2010). Multifunctional polymers for micro and nano patterned thin films. Polymer Preprints, 51, 211.
Wittenbeck, P., Franzke, D., & Wokaun, A. (1990). Photochemically induced breakdown of rod-like micelles: light-induced viscosity changes. Journal of Photochemistry and Photobiology A: Chemistry, 53, 343–358. DOI: 10.1016/1010-6030(90)87138-2.
Wolfe, J. P., & Buchwald, S. L. (1997a). Room temperature catalytic amination of aryl iodides. The Journal of Organic Chemistry, 62, 6066–6068. DOI: 10.1021/jo970876x.
Wolfe, J. P., & Buchwald, S. L. (1997b). Improved functional group compatibility in the palladium-catalyzed amination of aryl bromides. Tetrahedron Letters, 38, 6359–6362. DOI: 10.1016/s0040-4039(97)01463-9.
Yang, Z., & Cao, W. (2004). Self-assembliesof poly(4-diazonium styrene. Macromolecular Chemistry and Physics, 205, 241–246. DOI: 10.1002/macp.200300020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Robert Kerber on the occasion of his 90th birthday
Rights and permissions
About this article
Cite this article
Baranovicova, E., Stasko, A. & Nuyken, O. Diazene sulphonate as a cross-linking agent for polymers with pendant triarylamine hole-conducting units. Chem. Pap. 70, 1238–1252 (2016). https://doi.org/10.1515/chempap-2016-0063
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2016-0063