Abstract
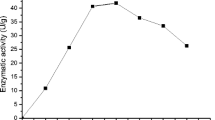
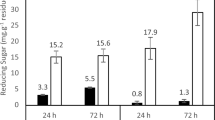
Cyclodextrin glycosyltransferase (CGTase) is an enzyme that produces cyclodextrins from starch by an intramolecular transglycosylation reaction. Cyclodextrins have been shown to have a number of applications in the food, cosmetic, pharmaceutical, and chemical industries. In the current study, the production of CGTase by Paenibacillus campinasensis strain H69-3 was examined in submerged and solid-state fermentations. P. campinasensis strain H69-3 was isolated from the soil, which grows at 45°C, and is a Gramvariable bacterium. Different substrate sources such as wheat bran, soybean bran, soybean extract, cassava solid residue, cassava starch, corn starch, and other combinations were used in the enzyme production. CGTase activity was highest in submerged fermentations with the greatest production observed at 48–72 h. The physical and chemical properties of CGTase were determined from the crude enzyme produced from submerged fermentations. The optimum temperature was found to be 70–75°C, and the activity was stable at 55°C for 1 h. The enzyme displayed two optimum pH values, 5.5 and 9.0 and was found to be stable between a pH of 4.5 and 11.0.
Similar content being viewed by others
References
Bender, H. (1986), Adv. Biotechnol. Process 6, 31–71.
Szejtli, J. (1997), J. Mater. Chem. 7, 575–587.
Tonkova, A. (1998), Enzyme Microb. Technol. 22 678–686.
Szejtli, J. (1982), Starch/Stärke 34, 379–385.
Pszczola, D.E. (1988), Food Technol January, 96–100.
Allegre, M. and Deratani, A. (1994), Agroo Food Ind. Hi Technol. January/February, 9–17.
Nakamura, N. and Horikoshi, K. (1976), Agric. Biol. Chem. 40, 1785–1791.
Nakamura, N. and Horikoshi, K. (1976), Agric. Biol. Chem. 40, 935–941.
Yu, E. K. C., Aoki, H., and Misawa, M. (1988), Appl. Microbiol. Biotechnol. 28, 377–379.
Tomita, K., Kaneda, M., Kawamura, K., and Nakanishi, K. (1993), J Fermen. Bioeng. 75, 89–92.
Bovetto, L. J., Backer, D. P., Villette, J. R., Sicard, P. J., and Bouquelet, S. J.-L. (1992), Biotechnol. Appl. Biochem. 15, 48–58.
Sabioni, J. G. and Park, Y. K. (1992), Starch/Stärke 44, 225–229.
Yim, D. G., Sato, H. H., Park, Y. H. E., and Park, Y. K. (1997), J. Ind. Microbiol. Biotechnol. 18, 402–405.
Matioli, G., Zanin, G. M., Guimarães, M. F., and Moraes, F. F. (1998), Appl. Biochem. Biotechnol. 70–72, 267–275.
Gawande, B. N., Singh, R. K., Chauhan, A. K., Goel, A., and Patkar, A. (1998), Enzyme Microb. Technol. 22, 288–291.
Martins, R. F. and Hatti-Kaul, R. (2002), Enzyme Microb. Technol. 30, 116–124.
Alves-Prado, H. F., Gomes, E., and DaSilva, R. (2002), Bol. SBCTA. 36, 43–54.
Chung, H. J., Yoon, S. H., Lee, M. J., et al. (1998), J. Agric. Food Chem. 46, 952–959.
Kabavainova, L., Dobreva, E., and Miteva, V. (1999), J. Appl. Microbiol 86, 1017–1023.
Bender, H. (1977), Arch. Microbiol. 111, 271–281.
Gawande, B. N. and Patkar, A. Y. (2001), Enzyme Microb. Technol. 28, 9, 10.
Mori, S., Hirose, S., Oya, T., and Kitahata, S. (1994), Biosc. Biotechnol. Biochem. 58, 1968–1972.
Larsen, K. L., Duedhal-Olisen, L., Christensen, H. J. S., Mathiesen, F., Pedersen, L. H., and Zimmermann, W. (1998), Carbohyd. Res. 310, 211–219.
Starnes, R. L. (1990), Cereal Foods World 35, 1094–1099.
Zamost, B. L., Nilsen, H. K., and Starnes, R. L. (1991), J. Ind. Microbiol. 8, 71–82.
Wind, R. D., Libl, W., Buitelaar, R. M., et al. (1995), Appl. Environ. Microbiol. 61, 1257–1265.
Tachibana, Y., Kuramura, A., Shirasaka, N., et al. (1997), Appl. Environ. Microbiol. 65, 1991–1997.
Raimbault, M. (1998), Eletr. J. Biotechnol. 1, 174–188.
Ramakrishna, S. V., Saswathi, N., Sheela, R., and Jamuna, R. (1994), Enzyme Microb. Technol. 16, 441–444.
Horikoshi, K. (1996) FEMS Microbiol. Rev. 18, 259–270.
Park, C. S., Park, K.H., and Kim, S. H. (1989), Agric. Biol. Chem. 53, 1167–1169.
Alves-Prado, H. F., Gomes, E., and DaSilva, R. (2002), Brazilian J Food Technol. 5, 189–196.
Dias, A. A. M., Andrade, C. M. M. C., and Linardi, V. R. (1992), Rev. Microbiol. 23, 189–193.
Fuwa, H. (1954), J. Biochem. 41, 583–603.
Pongsawasdi, P. and Yagisawa, M. (1987), J. Fermen. Technol. 65, 463–467.
Mäkelä, M. J., Korpela, T. K., Puisto, J., and Laakso, S. V. (1988), Agric Food Chem. 36, 83–88.
Sarath, G. (1996), In: Proteolytic Enzymes a Practical Approach. Beynon, R. J., Bond, J. S., (ed.), Oxford University Press, New York, pp. 25–55.
Hartree, E. F. (1972), Anal. Biochem. 48, 422–427.
Yoon, J.-H., Yim, D. K., Lee, J.-S., et al. (1998), Internat. J. System. Bacteriol. 48, 833–837.
Bailey, J. E. and Ollis, D. F. (1986), Biochemical Engineering Fundamentals. 2nd edition, McGraw-Hill International Editions, New York.
Damaso, M. C. T., Andrade, C. M. M. C., Pereira, N. Jr, (2000), Appl. Biochem. Biotechnol. 84, 821–834.
Pandey, A. (1992), Process Biochem. 27, 109–117.
Jamuna, R., Saswathi, N., Sheela, R., and Ramakrishna, S. V. (1993), Appl. Biochem. Biotechnol. 43, 163–176.
Salva, T. J. G., Lima, V. B., and Pagan, A. P. (1997), Rev. Microbiol. 28, 157–164.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves-Prado, H.F., Gomes, E. & Da Silva, R. Evaluation of solid and submerged fermentations for the production of cyclodextrin glycosyltransferase by Paenibacillus campinasensis H69-3 and characterization of crude enzyme. Appl Biochem Biotechnol 129, 234–246 (2006). https://doi.org/10.1385/ABAB:129:1:234
Issue Date:
DOI: https://doi.org/10.1385/ABAB:129:1:234