Abstract
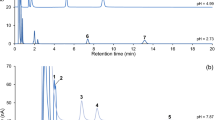
A GC-MS method was developed for measuring hydroxyl-radical capture products of salicylic acid, a common trapping agent for this reactive oxygen species, in samples obtained by in vivo cerebral microdialysis experiments. The assay employed liquid–liquid extraction followed by derivatization of 2,3- and 2,5-dihydroxybenzoic acid, along with 3,5-dihydroxybenzoic acid added as an internal standard. Due to their simple electron ionization mass spectra featuring [M–57]+ ions through the loss of tertiary alkyl group from the corresponding molecular ions, tert-butyldimethylsilyl (TBDMS) derivatives afforded straightforward method development based on selected-ion monitoring. In addition, tandem mass spectrometry probing collision-induced dissociation of [M–57]+ ions obtained from the isomeric tert-butyldimethylsilyl derivatives revealed characteristic differences in the resultant product-ion spectra. Our work has demonstrated the applicability of GC-MS for the assay of microdialysates for 2,3- and 2,5-dihydroxybenzoic acid by confirming that local administration of the excitotoxic glutamate into the rat striatum significantly increased in vivo hydroxyl-radical production in this brain region and that subsequent systemic administration of α-phenyl-tert-butylnitrone reversed glutamate-induced oxidative stress.





Similar content being viewed by others
References
Kaur H, Halliwell B (1996) In: Free radicals, a practical approach (Punchard NA, Kelly FJ, Eds), Oxford University Press, Oxford, pp 101–116
Nakao N, Brundin P (1998) Prog Brain Res 116:245–263. doi:10.1016/S0079-6123(08)60441-0
Tabner BJ, Turnbull S, El-Agnaf OMA, Allsop D (2002) Free Radic Biol Med 32:1076–1083. doi:10.1016/S0891-5849(02)00801-8
Halliwell B (2006) J Neurochem 97:1634–1658. doi:10.1111/j.1471-4159.2006.03907.x
Ferger B, Van Amsterdam C, Seyfried C, Kuschinsky K (1998) J Neurochem 70:276–280
Teismann P, Ferger B (2000) Brain Res Protoc 5:204–210. doi:10.1016/S1385-299X(00)00014-3
Themann C, Teismann P, Kuschinsky K, Ferger B (2001) J Neurosci Methods 108:57–64. doi:10.1016/S0165-0270(01)00370-3
Halliwell B, Kaur H, Ingelman-Sundberg M (1991) Free Radic Biol Med 10:439–441. doi:10.1016/0891-5849(91)90052-5
Molnar-Perl I, Horvath K, Bartha R (1998) Chromatographia 48:101–110. doi:10.1007/BF02467525
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Prokai L, Kim HS, Zharikova A, Roboz J, Ma L, Deng L et al (1998) J Chromatogr A 800:59–68. doi:10.1016/S0021-9673(97)01295-8
Thanikachalam S, Rajappan M, Kannappan V (2008) Chromatographia 67:41–47. doi:10.1365/s10337-007-0452-y
Ungerstedt U, Herrera-Marschitz M, Jungnelius U, Ståhle L, Tossman U, Zetterström T (1982) Adv Biosci 37:219–231
Okazaki S, Nishida Y, Kawai H, Saito S (1996) Neurochem Res 21:1201–1207. doi:10.1007/BF02532396
Weber GF (1999) Neurosci Biobehav Rev 23:1079–1086. doi:10.1016/S0149-7634(99)00041-X
Glantz SA (2002) Primer of Biostatistics, 5th edn. McGraw-Hill, New York, pp 110–111
Wan FJ, Tung CS, Shiah IS, Lin HC (2006) Eur Neuropsychopharmacol 16:147–153. doi:10.1016/j.euroneuro.2005.07.002
Prokai L, Prokai-Tatrai K, Perjesi P, Zharikova D, Perez J, Liu R et al (2003) Proc Natl Acad Sci USA 100:11741–11746. doi:10.1073/pnas.2032621100
Acknowledgments
Financial support for this work was provided in part by the grant NS044765 from the National Institute of Health (Bethesda, MD, USA), and the STARS program of the University of North Texas Health Science Center. Laszlo Prokai is the Robert A. Welch Professor of the University of North Texas Health Science Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, V., Bonds, D.V. & Prokai, L. Measurement of Hydroxyl-Radical Formation in the Rat Striatum by In Vivo Microdialysis and GC-MS. Chroma 68 (Suppl 1), 57–62 (2008). https://doi.org/10.1365/s10337-008-0703-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-008-0703-6