Abstract
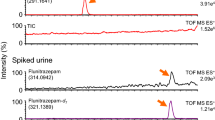
In bioanalytical LC-MS-MS matrix effects influencing the ionization process are a major concern with respect to the quality of the results obtained. In general such matrix effects are directly related to an insufficient sample clean-up of the biofluids. In order to establish a MS-adequate clean-up procedure for basic analytes present in biofluids (e.g. urine, plasma) which is based on solid-phase extraction (SPE) principles a combination of tailor-made SPE column packings and automated column-switching was developed. This novel, multidimensional (MD) SPE platform relies on the combination of a SPE column packed with a restricted access material (RAM) allowing size-exclusion and reversed phase chromatography (SEC-RPC) and a second SPE column packed with a mixed-mode phase (MMP) allowing ion exchange and reversed phase chromatography (IEX-RPC). For the evaluation of this MD-SPE platform 8 tricyclic antidepressants and two metabolites were chosen as model analytes. In order to monitor matrix effects, i.e. ion suppression, postcolumn infusion experiments were performed and compared with a two-dimensional SPE column mode (SEC-RPC). The MD-SPE platform is highly efficient for removal of low and high molecular weight sample components which suppress ionization to varying extend. In addition electrospray ionization of the model analytes is not affected by inter- or intra-individual variations in the composition of the matrix investigated. It is also independent of the species the biofluids originate from. It was demonstrated that the MD-SPE platform has a generic potential with respect to on-line SPE of basic drugs having a pKa > 6.5 and a moderate to low polarity and being present in different biofluids.
Similar content being viewed by others
References
Annesley TM (2003) Clin Chem 49: 1041–1044
Mallet CR, Lu Z, Mazzeo JR (2004) Rapid Commun Mass Spectrom 18: 49–58
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Anal Chem 75: 3019–3030
Taylor PJ (2005) Clin Biochem 38: 328–334
McCauley-Myers DL, Eichhold TH, Bailey RE (2000) J Pharmaceut Biomed Anal 23: 825–835
de Jager AD, Hundt HKL, Swart KJ, Hundt AF, Els J (2002) J Chromatogr B 773: 113–118
Lagerwerf FM, van Dongen WD, Steenvoorden RJJM, Honing M, Jonkman JHG (2000) Trends Anal Chem 19 (7): 418–427
Nassar AE, Varshney N, Getek T, Cheng L (2001) J Chromatogr Sci 39 (2): 59–64
Heinig K, Bucheli F (2002) J Chromatogr B 769: 9–26
Koal T, Deters M, Resch K, Kaever V (2006) Clinica Chimica Acta 364: 239–245
Foltz DJ, Castro-Perez J, Riley P, Entwisle JR, Baker TR, (2005) J Chromatogr B 825: 144–151.
Bourgogne E, Grivet C, Hopfgartner G (2005) J Chromatogr B 820: 103–110
Polson C, Sarkar P, Incledon B, Raguvaran V, Grant R (2003) J. Chromatogr. B 785: 263–275
Schoenmakers PJ, Marriott P, Beens J (2003) LCGC Eur 16: 335–339
Majors RE (2005) LCGC NA 23: 74–75
Majors RE, Boos KS, Grimm CH, Lubda D, Wieland G (1996) LCGC 14 (7): 554–559
Bonfiglio R, King RC, Olah TV, Merkle K (1999) Rapid Commun Mass Spectrom 13: 1175–1185
Boos KS, Rudolphi A, Vielhauer S, Walfort A, Lubda D, Eisenbeiß F (1995) Fresenius J Anal Chem 352: 684–690
Grimm CH, Boos KS (1999) Trends Anal Chem 18: 175–180
Boos KS, Fleischer CT (2001) Chimia 55 (1–2): 42–45
Vintiloiu A, Mullett WM, Papp R, Lubda D, Kwong E (2005) J Chromatogr A 1082 (2): 150–157
Schäfer C, Lubda D (2001) J Chromatogr A 909: 73–78
Rudolphi A, Boos KS (1997) LCGC 15 (9): 813–823
Boos KS, Rudolphi A (1997) LCGC 15 (7): 602–609
Bruins CHP, Jeronimus-Stratingh CM, Ensing K, van Dongen WD, de Jong GJ (1999) J Chromatogr A 863: 115–122
Blanca J, Munoz P, Morgado M, Mendez N, Aranda A, Reuvers T, Hooghuis H (2005) Anal Chimica Acta 529: 199–205
Georgi K, Boos KS (2004) LCGC Eur 17 (11A): 21–24
FDA (2001) Guidance for Industry – Bioanalytical Method Validation, CDER, CVM
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Georgi, K., Boos, K.S. Multidimensional On-Line SPE for Undisturbed LC-MS-MS Analysis of Basic Drugs in Biofluids. Chroma 63, 523–531 (2006). https://doi.org/10.1365/s10337-006-0804-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-006-0804-z