Summary
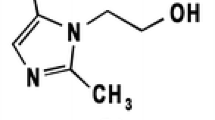
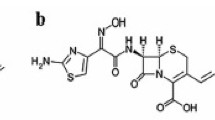
Conditions have been established for identification and quantification of cefuroxime axetil and cefuroxime by thin-layer chromatography and densitometry. Good separation of these compounds was achieved on silica gel by use of chloroform–ethyl acetate–glacial acetic acid–water, 4:4:4:1 (v/v), as mobile phase. UV densitometry was used to detect spots on chromatograms. Under these conditions the limits of detection for cefuroxime axetil and cefuroxime were 40 ng and 30 ng, respectively. Recoveries of cefuroxime axetil and cefuroxime were 99.93% and 97.94%, respectively.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krzek, J., Dąbrowska-Tylka, M. Simultaneous Determination of Cefuroxime Axetil and Cefuroxime in Pharmaceutical Preparations by Thin-Layer Chromatography and Densitometry. Chromatographia 58, 231–234 (2003). https://doi.org/10.1365/s10337-003-0039-1
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1365/s10337-003-0039-1