Abstract
Background
Many patients with clinically node-positive breast cancer receive neoadjuvant chemotherapy (NAC). Recent trials suggest the potential for limiting axillary surgery in patients who convert to pathologically node-negative disease. The authors developed a nomogram to predict axillary response to NAC in patients with cN1 disease that can assist clinicians in treatment planning.
Methods
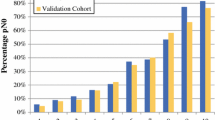
Patients with cT1–4N1M0 breast cancer who received NAC and underwent axillary lymph node dissection from 2001 through 2013 were identified (n = 584). Uni- and multivariate logistic regression analyses were performed to determine factors predictive of nodal conversion. A nomogram to predict the likelihood of nodal pathologic complete response (pCR) was constructed based on clinicopathologic variables and validated using an external dataset.
Results
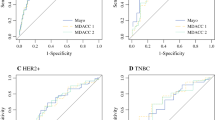
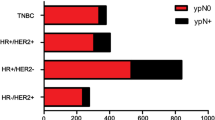
Axillary pCR was achieved for 217 patients (37 %). Patients presenting with high nuclear grade [grade 3 vs. 1, odds ratio (OR) 13.4], human epidermal growth factor receptor 2-positive (OR 4.7), estrogen receptor (ER)-negative (OR 3.5), or progesterone receptor-negative (OR 4.3) tumors were more likely to achieve nodal pCR. These factors, together with clinically relevant factors including presence of multifocal/centric disease, clinical T stage, and extent of nodal disease seen on regional nodal ultrasound at diagnosis were used to create nomograms predicting nodal conversion. The discrimination of the nomogram using ER+ status (>1 % staining) versus ER− status [area under the curve (AUC) 78 %] was improved slightly using the percentage of ER staining (AUC 78.7 %). Both nomograms were validated using an external cohort.
Conclusion
Nomograms incorporating routine clinicopathologic parameters can predict axillary pCR in node-positive patients receiving NAC and may help to inform treatment decisions.


Similar content being viewed by others
References
Kuerer H, Sahin A, Hunt K, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230:72–8.
Buzdar A, Ibrahim N, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–84.
Hennessy B, Hortobagyi G, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–11.
Dominici L, Negron Gonzalez V, Buzdar A, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116:2884–9.
Boughey J, Suman V, Mittendorf E, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61.
Rouzier R, Extra J, Klijanienko J, et al. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20:1304–10.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258–64.
Caudle A, Yang W, Mittendorf E, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg. 2014;34:1072–8.
Mittendorf E, Caudle A, Yang W, et al. Implementation of the American College of Surgeons Oncology Group Z1071 trial data in clinical practice: Is there a way forward for sentinel lymph node dissection in clinically node-positive breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014;21:2468–73.
Krishnamurthy S, Sneige N, Bedi D, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–8.
Edge S, Byrd D, Compton C. AJCC cancer staging manual. 7th ed. Springer, New York; 2009.
Caudle A, Yu T, Tucker S, et al. Local–regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83.
Kakuda J, Stuntz M, Trivedi V, Klein S, Vargas H. Objective assessment of axillary morbidity in breast cancer treatment. Am Surg. 1999;65:995–8.
Lucci A, McCall A, Beitsch P, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63.
Fleissig A, Fallowfield L, Langridge C, et al. Postoperative arm morbidity and quality of life: results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–93.
Bear H, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74.
Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local–regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–93.
Alvarado R, Yi M, Le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19:3177–83.
Classe J, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009;27:726–32.
Newman E, Sabel M, Nees A, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol. 2007;14:2946–52.
Mamounas E, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–702.
Caudle A, Yang W, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–8.
Boughey J, Ballman K, Le-Petross H, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263:802–7.
Choy N, Lipson J, Porter C, et al. Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann Surg Oncol. 2015;22:377–82.
Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261:378–82.
Caudle A, Yang W, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;10:1072–8.
Boughey J, McCall L, Ballman K, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260:608–14.
Schipper R, Moossforff M, Nelemens P, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno)therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer. 2014;14:315–22.
Hammond M, Hayes D, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–22.
Giuliano A, Hawes D, Ballman K, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306:385–93.
Weaver D, Ashikaga T, Krag D, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412–21.
Boughey J, Ballman K, Symmans W, et al. Methods impacting the false-negative rate of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0–T4, N1–2) who receive neoadjuvant chemotherapy: results from a prospective trial—ACOSOG Z1071 (Alliance). In: San Antonio breast cancer symposium 2014, Poster Presentation, 2014. http://eposter.abstractsonline.com/sabcs. Accessed 31 Jan 2015.
Acknowledgment
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672). Dr. Jose Vila is supported by a Grant from Umberto Veronesi Foundation and the Carlos III Health Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material. Supplemental Fig. 1. Nomogram for predicting axillary pathologic complete response after neoadjuvant chemotherapy using estrogen receptor categorized as positive (>1 % of the cells staining) or negative (0 % of cells staining) without using the number of abnormal nodes on US. The unadjusted AUC is 77.6 %
Rights and permissions
About this article
Cite this article
Vila, J., Mittendorf, E.A., Farante, G. et al. Nomograms for Predicting Axillary Response to Neoadjuvant Chemotherapy in Clinically Node-Positive Patients with Breast Cancer. Ann Surg Oncol 23, 3501–3509 (2016). https://doi.org/10.1245/s10434-016-5277-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5277-1