Abstract
Background
The role of ultrasound examination in detection of postprocedure complications from totally implantable venous access devices (TIVAD) placement is still uncertain. In a cohort of 665 cancer outpatients, we assessed a quick ultrasound examination protocol in early detection of mechanical complications of catheterization.
Methods
Immediately after TIVAD placement, an ultrasound examination and chest radiography were performed to detect hemothorax, pneumothorax, and catheter malposition. The two methods were compared.
Results
Of the 668 catheters inserted, 628 were placed into axillary veins and 40 into internal jugular veins. The ultrasound examination took 2.5 ± 1.1 min. No hemothorax was detected, and neither pneumothorax nor catheter malposition was evident among the 40 internal jugular vein cannulations. Ultrasound and chest radiography examinations of the 628 axillary vein cannulations detected five and four instances of pneumothorax, respectively. Ultrasound detected all six catheter malpositions into the internal jugular vein. However, ultrasound failed to detect two out of three malpositions in the contralateral brachiocephalic vein and one kinking inside the superior vena cava. Without revision surgery, the operating time was 34.1 ± 15.6 min. With revision surgery, the operating time was shorter when ultrasound detected catheter malposition than when chest radiography was used (96.8 ± 12.9 vs. 188.8 ± 10.3 min, p < 0.001).
Conclusions
Postprocedure ultrasound examination is a quick and sensitive method to detect TIVAD-related pneumothorax. It also precisely detects catheter malposition to internal jugular vein thus reduces time needed for revision surgery while chest radiography remains necessary to confirm catheter final position.
Similar content being viewed by others
Totally implantable venous access devices (TIVAD) are mandatory to oncology patients for the administration of chemotherapeutic agents with better venous access reliability. It has become more popular to place TIVAD by the Seldinger technique via venipuncture because of the shorter duration of the procedure and the possibility of switching from a procedure requiring an operating theater to a less costly outpatient procedure.1 However, venipuncture for TIVAD placement can sometimes be associated with serious complications in cancer patients. Compared with the anatomical landmark directed puncture, ultrasound-assisted technique for central venous catheter (CVC) placement is promoted for safer and a lower placement failure rate properties.2–8 Despite the global acceptance of ultrasound-assisted CVC and TIVAD placement, the role of ultrasound examination to detect TIVAD placement-related mechanical complications is uncertain and has not been fully investigated.
In our institute, there were 800–1000 TIVAD placements for cancer outpatients by the ultrasound-assisted percutaneous Seldinger technique annually. To maintain an efficient and safe work flow and to avoid sending patients home with undetected complications, we have been performing quick ultrasound examinations immediately after TIVAD placement to detect postprocedure mechanical complications.
In this prospective study, a large series of ultrasound examinations in a cohort of cancer outpatients who underwent TIVAD placement was analyzed to determine whether ultrasound could facilitate and speed up detection of several mechanical complications of catheter placement such as hemothorax, pneumothorax, and catheter malposition. To accelerate the pace of identification, we used the same ultrasound transducer for both TIVAD placement and the postprocedure examination.
Materials and Methods
This prospective, observational study was carried out in a tertiary-care university-affiliated medical center. We obtained protocol approval from the Research Ethics Committee of our institute (NTUH-201004048R) and informed consent from each patient. We conducted the ultrasound examination immediately after TIVAD placement surgery in 665 consecutive cancer outpatients. Anesthesiology residents and attending physicians (who had placed at least 10 TIVAD independently) were the TIVAD placement surgeons. Residents were supervised by an experienced attending physician.
Surgical Procedure
All patients were sedated, with spontaneous ventilation maintained during the procedure. Each patient was placed in the Trendelenburg position with neck slightly extended, head turned contralateral to the surgical side, and arms adducted. We chose the axillary vein for TIVAD placement because it lies entirely outside the thoracic cage, thereby decreasing the risk of pneumothorax and hemothorax. The longitudinal axis of the axillary vein was identified by ultrasound (Sonosite, MicroMaxx System with a 6–13 MHz linear transducer). The ultrasound scanning and operation procedures have been previously described.9 The catheter length was measured as the distance of the assumed course of the vein from the implanted port pocket to the lateral border of the sternum at the level between the third and fourth ribs. After two failed puncture attempts to enter the axillary vein or inadvertent axillary artery puncture, the attending physician decided whether to shift to real-time ultrasound-guided internal jugular vein catheterization or to continue axillary vein cannulation.
Postoperative Examination and Management
After completing the TIVAD placement and before sending the patient to the postanesthesia care unit (PACU), an ultrasound examination was performed by a single examiner who underwent 2 h′ training in point-of-care ultrasound targeted to specific mechanical complications of CVC placement described as bellow section before initiation of this investigation. Each patient was scanned while in the supine position, with the head in the neutral position. We used the same 6–13 MHz linear transducer as used in TIVAD placement. The examination procedure was as follows.
The probe was placed in the precordial area (third or fourth parasternal intercostal space). Sliding lung sign with comet tail sign were noted to rule out pneumothorax. A seashore sign or sandy pattern on M-mode was used to further confirm the absence of pneumothorax. The presence of the stratosphere sign on M-mode was indicative of pneumothorax.10
The probe was placed at the third or fourth intercostal space around the mid to posterior axillary line to detect the presence of pleural effusion or hemothorax.
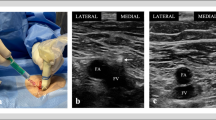
The probe was placed at the lateral supraclavicular fossa, tilted toward the thoracic cavity, and slowly moved to the medial supraclavicular fossa with a frequent tilting maneuver to observe the catheter inside the subclavian vein, turned toward the brachiocephalic vein, and finally toward the mediastinum (Fig. 1; Supplemental Fig. 1; Supplemental Video 1).
An ultrasound scan from the brachiocephalic vein back to the internal jugular vein (IJV) was performed to document that no catheter was present inside the IJV (Supplemental Video 2). If found in the IJV, the catheter was considered to be malpositioned (Supplemental Video 3).
The supraclavicular fossa and IJV were scanned on the contralateral side to detect catheter malposition to the contralateral side.
Postprocedure chest radiographs were arranged in the PACU and were all reviewed by board-certified attending radiologists for catheter tip localization and the presence or absence of hemopneumothoraces.
When pneumothorax was diagnosed either by ultrasound or chest radiographs, supplemental oxygen was provided and the patient’s length of PACU stay was extended. A chest radiograph was performed 2–4 h later. If the pneumothorax amount was small and did not progress further, the patient was discharged with instructions. If it was clinically significant or progressed, the patient was admitted with placement of a chest tube, if clinically indicated.
Revision surgery was indicated if the catheter was malpositioned outside the superior vena cava (SVC) or upper right atrium. If catheter malposition was diagnosed by ultrasound in the operating room, revision surgery was performed immediately before sending the patient to the PACU. If catheter malposition was diagnosed in the PACU by chest radiograph, the patient was sent back to the operating room for revision surgery.
Outcome Measurement
Patient demographic data, including age, sex, and types of cancers, were collected. To detect mechanical complications such as hemothorax, pneumothorax, and malposition of the catheter, each patient received an ultrasound examination immediately after surgery and a chest radiograph examination after being sent to the PACU. The time needed for catheter repositioning after ultrasound examination (immediate revision surgery) or postprocedurally after chest radiography (delayed revision surgery) was compared. The accuracies of the ultrasound and chest radiograph examinations were compared. By reviewing standing posterior–anterior view chest radiographs (images taken during patients’ subsequent follow-up visit, not the image taken in the PACU), the final catheter tip location was evaluated by an independent examiner (Dr. F. S. Lin). The final catheter tip location was defined according to the following criteria (Supplemental Fig. 2): A, catheter tip located within 1 cm above or below the caval–atrial junction; B, catheter tip located within 2 cm below the lower end of position A; C, catheter tip located below position B but still within the right atrium; and D, catheter tip located above the upper end of position A but still within the SVC. Tip location at position A or B was considered optimal; catheter tip location at position C or D was defined as suboptimal.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or number (proportion). The time needed to complete catheter repositioning after the ultrasound examination (immediate revision surgery) or postprocedurally after chest radiography (delayed revision surgery) was compared by Student’s t test. The results of final catheter tip location between different approach sites were compared by the Chi square test or Fisher’s exact test, as appropriate. Statistical significance was defined as a p value of less than 0.05. All statistical analysis was performed by SigmaPlot for Windows, version 12 (SAS Institute, Cary, NC, USA).
Results
The patients’ characteristics and cancer diagnoses are summarized in Table 1. The most common types of cancer diagnosed in our patient population were genitourinary cancer (23.0 %), lung cancer (21.9 %), and hematological malignancy (21.7 %). The placement of 668 catheters in 665 patients was evaluated during the study period. A total of 628 TIVAD (including 3 revision surgeries) were placed via axillary vein cannulation (right, 531; left, 97); 40 catheters shifted access site from the axillary vein to the IJV because of more than two failed puncture attempts (right, 35; left, 5).
Catheter-related Complications and Ultrasound Examination Results
The mean time needed to perform the ultrasound examination was 2.5 ± 1.1 min. The time needed for examination of the first 100 patients was not significantly different from those of the other patients during the later investigation period. Hemothorax was not detected in our study population. Ultrasound detected pneumothorax in five patients (0.7 %) receiving right axillary vein cannulation. Two pneumothoraces were considered clinically insignificant. After observing both patients for an extended period and providing supplemental oxygen in the PACU, chest radiographs were performed and showed no progression of their conditions. Both patients were discharged with instructions. In one of these two patients, the subtle change detected by ultrasonography was undetectable by chest radiography (Fig. 2). Admission to the hospital was indicated in the other three patients with clinically significant pneumothorax. A chest tube was inserted in one patient, and pigtail drains were inserted in the other two patients.
Ten catheters (1.5 %) were malpositioned in our study population. All of them were in the axillary vein cannulation group (total 628 catheters, including three revision surgeries). There were six catheters in the ipsilateral IJV, three in the contralateral brachiocephalic vein, and one kinked catheter in the SVC. Ultrasound detected all six malpositioned catheters in the IJV, including one catheter with a loop extending into the base of the IJV (Fig. 3a). A radiograph taken in the operating room confirmed the catheter looping (Fig. 3b). The catheters of all six patients were repositioned while in the operating room. However, ultrasound detected only one of the three malpositioned catheters into contralateral brachiocephalic vein and failed to detect catheter kinking inside the SVC (Supplemental Fig. 3). Chest radiograph detected these malpositioned catheters in the PACU. These three patients were sent back to the operating room for delayed revision surgery. The mean time to complete surgery without revision, with immediate revision, and with delayed revision (in minutes) was 34.1 ± 15.6, 96.8 ± 12.9, and 188.8 ± 10.3, respectively. It was significantly shorter for surgery with immediate revision than for with delayed revision (p < 0.001).
In the 40 patients receiving real-time ultrasound-assisted IJV cannulations, neither pneumothorax nor catheter malposition occurred.
Final Catheter Tip Location
The final catheter tip location on follow-up chest radiograph is summarized in Table 2. There were 95.6 % (505 of 528) of right axillary vein cannulated catheters located at optimal position, which was comparable to right IJV cannulated catheters (94.3 %, 33 of 35, p = 0.71). However, there were 90.7 % (88 of 97) of left axillary vein cannulated catheters located at optimal position, which is significantly less than the right axillary vein approach (p = 0.043). The rate of optimal tip location for left IJV (60 %, 3 of 5) cannulation was even less than left axillary vein approach (p = 0.031).
Discussion
To our knowledge, ours is the first large series to indicate that fast ultrasound examination can facilitate early detection of ultrasound-assisted TIVAD placement-related mechanical complications.
In a recent meta-analysis of percutaneous subclavian vein puncture versus surgical venous cutdown for the placement of TIVAD in cancer patients, ultrasound-assisted percutaneous TIVAD placement was advocated because it has a lower implantation failure rate. However, serious complications occurred less frequently in surgical cutdown patients.11 Because most TIVAD placement is now arranged on an outpatient basis, it would be prudent to identify every potentially fatal placement-related mechanical complication before sending patients home.1,12 In our study, postprocedure chest radiography failed to detect one small-volume pneumothorax. Early ultrasound detection prevented us from sending this patient home and risking pneumothorax progression. Compared with chest radiography, ultrasound is a more sensitive and specific means of pneumothorax detection.13 However, using ultrasound to detect pneumothorax is reported less during the perioperative period. In fact, thoracic ultrasound is convenient for pneumothorax detection in intraoperative settings.14 Our data showed that although the incidence of pneumothorax associated with ultrasound-assisted percutaneous TIVAD placement is very low (0.7 %), using the same ultrasound transducer for both percutaneous TIVAD placement and complication detection increases efficiency (average less than 3 min for the whole examination) and thus improves patient safety.
Postprocedure chest radiography is still necessary for catheter tip position confirmation by our protocol. Malposition of the catheter was found to occur more commonly after subclavian cannulation (especially via the right subclavian approach) than after IJV cannulation.15 Our data revealed that 60 % of the malpositioned catheters were located in the ipsilateral internal jugular vein, which is compatible with the finding of the previous report.15 It is difficult using ultrasound to locate catheters in other sites including the deep contralateral brachiocephalic vein or SVC owing to poor visibility inside the thoracic cage. Therefore, in our report, ultrasound only detected one out of three catheters malpositioned into contralateral brachiocephalic vein, though the contralateral internal jugular and subclavian veins were checked routinely. As expected, ultrasound did not detect catheter kinking within the SVC. Delayed revision surgery was indicated for malpositioned catheters undiagnosed by ultrasound. Still, most malpositioned catheters were to the internal jugular vein, and early detection by ultrasound resulted in immediate revision surgery that took 90 min off the time required for delayed revision surgery (96.8 vs. 188.8 min).
Fluoroscopy is one of the most common method to confirm catheter tip position.1 The advantage of fluoroscopy over ultrasound examination is a better confirmation of catheter tip position. However, fluoroscopy is associated with a small radiation exposure to both patients and care providers. Further limitation includes the need of expensive equipment that may not be always available (including in our institute). From our study result, 94.6 % (629 of 665) of final catheter tip location in our study group was optimal. However, further analysis revealed that left axillary vein cannulated TIVAD was slightly less precise than right axillary vein TIVAD (90.7 vs. 95.6 %), given the longer catheter length needed for left-side approach. We had only five cases of left IJV cannulated TIVAD, which was least precise in terms of final catheter tip position. There was only 60 % of left IJV TIVAD catheter tips located at an optimal position. The catheter of left IJV TIVAD has to travel from precordial port pocket site to subcutaneous tunnel to left neck incision to left IJV to left brachiocephalic vein to SVC, then finally to the caval–atrial junction and possibly the right atrium, which is the longest tract among the four different access sites. Without a fluoroscope guide, it is theoretically more difficult to estimate the catheter length needed only by surface landmark. We have considered integrating intravascular electrocardiograms into our future protocol because it has been reported to be a useful method to assess catheter tip position without the above-mentioned fluoroscopy-related shortcomings.16,17 However, in patients without a clear P wave, such as atrial fibrillation or catheter malposition into the affluent veins of the SVC precludes the definite catheter tip position by the intravascular electrocardiogram changes.
There is 94.3 % of right IJV cannulated TIVAD catheter tip located at optimal position, which is comparable to right axillary vein TIVAD. Moreover, there is neither catheter malposition nor pneumothorax detected in right IJV TIVAD group. We did not choose IJV as our primary access site because compared to a single-incision transpectoral axillary vein approach, the IJV approach requires two incisions, one standard precordial port site incision and one small neck incision. In addition, a subcutaneous tunnel from neck to chest is also necessary for the IJV approach. The IJV approach is generally more painful than the axillary approach.
From the patient’s perspective, there will be some cosmetic concerns from the subcutaneous tunnel for IJV-approach TIVAD. The subcutaneous tunnel looks prominent, especially when patients are cachetic. In addition, theoretically, the catheter inside the subcutaneous tunnel above clavicle for IJV TIVAD will limit patients in wearing heavy backpacks, and it may impair their daily activity functions and quality of life.
Three previous reports investigated the diagnostic value of ultrasound examinations after CVC placement; most patients were in intensive care units.18–20 Yet the implications of ultrasound examination have not been investigated in outpatients. Additionally, these previous three reports had far fewer patients [85 catheter insertions in 81 patients in one report; 83 catheter insertions including 41 peripherally inserted central catheters (PICCs) in 69 patients in another report; and 99 catheter insertions in 111 patients with 12 failures to perform ultrasound examinations]. In the previous three reports, most catheters were placed using the landmark-guided technique; only 12 CVCs (4.5 %) and 41 PICCs (15.4 %) among a total of 267 catheters were placed using the ultrasound-assisted technique. Because ultrasound-assisted CVC placement is associated with a lower incidence of complications, a larger number of patients will be necessary for the determination of the diagnostic value of the ultrasound examination after catheter insertion. To our knowledge, our study is currently the only one to investigate this issue in a large series of oncology outpatients.
This report had some limitations. First, ultrasound screening of the catheter tip on the right side of the heart was not performed in our patients for several reasons. Several clinical studies have demonstrated that the performance and durability of central venous catheters is improved if the tip is positioned at caval–atrial junction or within the right atrium.21–23 Because durability of the TIVAD is of primary concern in cancer patients who require long-term chemotherapy, a catheter tip located at the caval–atrial junction to the upper right atrium was considered to be an optimal position in our protocol. Moreover, our aim was to establish a protocol that facilitates work flow by allowing the same ultrasound transducer to be used for both catheter placement and evaluation of complications. Change to a sector probe is required for good cardiac examination performance. This was not only time-consuming but also not feasible because sector probes were unavailable in our outpatient surgery center. Ultrasound is reported to be better than chest radiography for confirmation of the catheter’s position in the right atrium.20 Chest radiography may be responsible for up to 47 % of falsely positive intra-atrial CVC tip placement results.24 Clinicians should be able to determine whether a cardiac ultrasound examination is needed in their own protocol. Intravascular electrocardiogram may also be considered. Second, the ultrasound examinations were performed by a single examiner and are operator dependent. However, the examiner only underwent 2 h’ training before this investigation, and the time needed for examination was not significantly different between the early and late investigation periods. This kind of examination is a point-of-care ultrasound application, allowing findings to be directly correlated with the patient’s presenting signs and symptoms.25 It is also qualitative, targeted to specific complications of CVC placement, easy to learn, and easily repeatable.
In summary, this is the first large series study conducted in an outpatient setting to indicate that a quick ultrasound examination can facilitate early and sensitive detection of ultrasound-assisted TIVAD placement-related mechanical complications, especially pneumothorax. Although the findings do not exclude the necessity of postprocedure chest radiography to confirm final tip position, immediate postprocedure ultrasound examinations can precisely detect catheter malposition into ipsilateral IJV and reduce the time needed for revision surgery.
References
Biffi R, Toro A, Pozzi S, Di Carlo I. Totally implantable vascular access devices 30 years after the first procedure. What has changed and what is still unsolved? Support Care Cancer. In press.
Troianos CA, Hartman GS, Glas KE, et al. Special articles: guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. 2012;114:46–72.
Rupp SM, Apfelbaum JL, Blitt C, et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116:539–73.
Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intens Care Med. 2012;38:1105–17.
Sekiguchi H, Tokita JE, Minami T, Eisen LA, Mayo PH, Narasimhan M. A prerotational, simulation-based workshop improves the safety of central venous catheter insertion: results of a successful internal medicine house staff training program. Chest. 2011;140:652–8.
Froehlich CD, Rigby MR, Rosenberg ES, et al. Ultrasound-guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Crit Care Med. 2009;37:1090–6.
Milling TJ, Jr., Rose J, Briggs WM, et al. Randomized, controlled clinical trial of point-of-care limited ultrasonography assistance of central venous cannulation: the Third Sonography Outcomes Assessment Program (SOAP-3) trial. Crit Care Med. 2005;33:1764–9.
Wu SY, Ling Q, Cao LH, Wang J, Xu MX, Zeng WA. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118:361–75.
Lin CP, Wang YC, Lin FS, Huang CH, Sun WZ. Ultrasound-assisted percutaneous catheterization of the axillary vein for totally implantable venous access device. Eur J Surg Oncol. 2011;37:448–51.
Piette E, Daoust R, Denault A. Basic concepts in the use of thoracic and lung ultrasound. Curr Opin Anaesthesiol. 2013;26:20–30.
Orci LA, Meier RP, Morel P, Staszewicz W, Toso C. Systematic review and meta-analysis of percutaneous subclavian vein puncture versus surgical venous cutdown for the insertion of a totally implantable venous access device. Br J Surg. 2014;101:8–16.
Duszak R Jr, Bilal N, Picus D, Hughes DR, Xu BJ. Central venous access: evolving roles of radiology and other specialties nationally over two decades. J Am Coll Radiol. 2013;10:603–12.
Wilkerson RG, Stone MB. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17:11–7.
Ueda K, Ahmed W, Ross AF. Intraoperative pneumothorax identified with transthoracic ultrasound. Anesthesiology. 2011;115:653–5.
Pikwer A, Baath L, Davidson B, Perstoft I, Akeson J. The incidence and risk of central venous catheter malpositioning: a prospective cohort study in 1619 patients. Anaesth Intensive Care. 2008;36:30–7.
Pawlik MT, Kutz N, Keyl C, Lemberger P, Hansen E. Central venous catheter placement: comparison of the intravascular guidewire and the fluid column electrocardiograms. Eur J Anaesthesiol. 2004;21:594–9.
Stas M, Mulier S, Pattyn P, Vijgen J, De Wever I. Peroperative intravasal electrographic control of catheter tip position in access ports placed by venous cut-down technique. Eur J Surg Oncol. 2001;27:316–20.
Matsushima K, Frankel HL. Bedside ultrasound can safely eliminate the need for chest radiographs after central venous catheter placement: CVC sono in the surgical ICU (SICU). J Surg Res. 2010;163:155–61.
Maury E, Guglielminotti J, Alzieu M, Guidet B, Offenstadt G. Ultrasonic examination: an alternative to chest radiography after central venous catheter insertion? Am J Respir Crit Care Med. 2001;164:403–5.
Vezzani A, Brusasco C, Palermo S, Launo C, Mergoni M, Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38:533–8.
Schutz JC, Patel AA, Clark TW, et al. Relationship between chest port catheter tip position and port malfunction after interventional radiologic placement. J Vasc Interv Radiol. 2004;15:581–7.
Vesely TM. Central venous catheter tip position: a continuing controversy. J Vasc Interv Radiol. 2003;14:527–34.
Mandolfo S, Galli F, Costa S, Ravani P, Gaggia P, Imbasciati E. Factors influencing permanent catheter performance. J Vasc Access. 2001;2:106–9.
Reynolds N, McCulloch AS, Pennington CR, MacFadyen RJ. Assessment of distal tip position of long-term central venous feeding catheters using transesophageal echocardiology. JPEN . 2001;25:39–41.
Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–57.
Acknowledgment
Financially supported by Department of Anesthesiology, National Taiwan University Hospital.
Disclosure
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2014_4222_MOESM1_ESM.jpg
Supplementary Fig. 1a. A scan from the lateral supraclavicular fossa traces the catheter’s path from the subclavian vein into the brachiocephalic vein (JPG 2158 kb)
10434_2014_4222_MOESM2_ESM.jpg
Supplementary Fig. 1b. A scan from the medial supraclavicular fossa traces the catheter’s path from the subclavian vein into the brachiocephalic vein (JPG 1911 kb)
10434_2014_4222_MOESM3_ESM.jpg
Supplementary Fig. 2. Definition of final catheter tip location. A: Catheter tip located within 1–cm above or below caval-atrial junction. B: Catheter tip located within 2–cm below lower end of position A. C: Catheter tip located below position B but still within right atrium. D: Catheter tip located above upper end of position A but still with in SVC. Tip location at position A or B was considered optimal. Once the catheter tip located at position C or D was defined as suboptimal (JPG 872 kb)
Supplemental Video 1. Trace the catheter from subclavian vein to brachiocephalic vein and observe the catheter travel toward mediastinum (AVI 592 kb)
Supplemental Video 2. Trace from internal jugular vein to brachiocephalic vein to document that there is no catheter malposition (AVI 659 kb)
Supplemental Video 3. Document a catheter malposition to internal jugular vein (AVI 557 kb)
Rights and permissions
About this article
Cite this article
Wu, CY., Lin, FS., Wang, YC. et al. Fast Track Ultrasound Protocol to Detect Acute Complications After Totally Implantable Venous Access Device Placement. Ann Surg Oncol 22, 1943–1949 (2015). https://doi.org/10.1245/s10434-014-4222-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4222-4