Abstract
Background
Isolated hepatic perfusion (IHP) with melphalan is an established approach for patients with unresectable metastatic liver lesions. This study determined the safety and maximum tolerated dose (MTD) of 5-FU with oxaliplatin via IHP.
Methods
Standard 3 × 3 Phase I design. Subjects with unresectable isolated CRC liver metastases scheduled for HAI pump were eligible. IHP used fixed-dose oxaliplatin with escalating 5-FU doses. Toxicity (CTCAE v 4.0) and response (RECIST), progression-free survival, and overall survival (OS) were assessed. Systemic and IHP plasma PK of 5-FU, anabolites, and platinum were determined.
Results
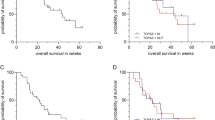
All 12 patients had received ≥1 line of pre-IHP chemotherapy. There were 4 grade 3 serious adverse events (33.3 %) and 1 grade 4 event (8.3 %). Also, 2 dose-limiting toxicities occurred at DL2 at 300 mg/m2, resulting in expansion of DL1 at 200 mg/m2 5-FU, the eventual MTD. At 6-month follow-up, 9 patients (82 %) demonstrated partial response, while 2 (18 %) exhibited stable disease. Also, 64 % of patients demonstrated a >50 % decrease in CEA. The 1- and 2-year OS probabilities were 90.9 and 71.6 %, respectively, with median follow-up of 24 months. IHP exposures (AUC0–60 min) were 10.9 ± 4.5 μgPt h/mL, 49.3 ± 30.7 μg h/mL 5-FU (DL1), and 70.5 ± 35.5 μg h/mL 5-FU (DL2). Systemic exposure (AUC0–inf) relative to IHP exposure was negligible for both platinum (1.1 ± 1.5 %) and 5-FU (0.09 ± 0.10 %).
Conclusions
The MTD for IHP was 200 mg/m2 5-FU with 40 mg/m2 oxaliplatin. Systemic exposure to the agents was minimal during IHP. The response and survival observed warrants assessment in a larger phase II trial.


Similar content being viewed by others
References
Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional versus systemic continuous 5 fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206:685–93.
Hohn DC, Stagg RJ, Friedman MA, Hannigan JF Jr., Rayner A, Ignoffo RJ, et al. A randomized trial of continuous intravenous versus hepatic intraarterial floxuridine in patients with colorectal cancer metastatic to the liver: The Northern California Oncology Group trial. J Clin Oncol. 1989;7:1646–54.
Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987;107:459–65.
Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481). J Clin Oncol. 2006;24:1395–403.
Lorenz M, Muller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–54.
Fiorentini G, Cantore M, Rossi S, Vaira M, Tumolo S, Dentico P, et al. Hepatic arterial chemotherapy in combination with systemic chemotherapy compared with hepatic arterial chemotherapy alone for liver metastases from colorectal cancer: results of a multi-centric randomized study. In Vivo. 2006;20:707–9.
Kemeny N. Management of liver metastases from colorectal cancer. Oncology. 2006;20:1161–76, 1179.
Alexander HR, Bartlett DL, Libutti SK. Isolated hepatic perfusion: a potentially effective treatment for patients with metastatic or primary cancers confined to the liver. Cancer J Sci Am. 1998;4:2–11.
Alexander HR, Bartlett DL, Libutti SK, Fraker DL, Moser T, Rosenberg SA. Isolated hepatic perfusion with tumor necrosis factor and melphalan for unresectable cancers confined to the liver. J Clin Oncol. 1998;16:1479–89.
Bartlett DL, Libutti SK, Figg WD, Fraker DL, Alexander HR. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery. 2001;129:176–87.
Alexander HR, Bartlett DL, Fraker DL, Libutti SK, Moser T, Rosenberg SA. Results of a Phase II study of isolated hepatic perfusion (IHP) with tumor necrosis factor (TNF) and melphalan for unresectable primary or metastatic cancer confined to the liver. Proc Soc Surg Oncol. 1997;6:8.
Alexander HR, Libutti SK, Bartlett DL, Puhlmann M, Fraker DL, Bachenheimer LC. A phase I–II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2000;6:3062–70.
Rothbarth J, Pijl ME, Vahrmeijer AL, Hartgrink HH, Tijl FG, Kuppen PJ, et al. Isolated hepatic perfusion with high-dose melphalan for the treatment of colorectal metastasis confined to the liver. Br J Surg. 2003;90:1391–7.
Zeh HF 3rd, Brown CK, Holtzman MP, Egorin MJ, Holleran JL, Potter DM, et al. A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2009;16(12):385–94.
Raymond E, Faivre S, Chaney S, Woynarowski J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther. 2002;1(3):227–35.
Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs. 199;8(9):876–85.
Kosovec JE, Egorin MJ, Gjurich S, Beumer JH. Quantitation of 5-fluorouracil (5-FU) in human plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:224–30.
Beumer JH, Parise RA, Egorin MJ, Newman EM, Doroshow JH, Synold TW, et al. Concentrations of the DNA methyltransferase inhibitor 5-fluoro-2′-deoxycytidine (FdCyd) and its cytotoxic metabolites in plasma of patients treated with FdCyd and tetrahydrouridine (THU). Cancer Chemother Pharmacol. 2008;62:363–8.
Colville H, Dzadony R, Kemp R, Stewart S, Zeh HJ 3rd, Bartlett DL, et al. In vitro circuit stability of 5-fluorouracil and oxaliplatin in support of hyperthermic isolated hepatic perfusion. J Extra Corpor Technol. 2010;42:75–9.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18.
Aigner KR, Walter H, Link KH. Isolated liver perfusion with MMC/5-FU: surgical technique, pharmacokinetics, clinical results. Contrib Oncol. 1988;29:229–46.
Schwemmle K, Link KH, Rieck B. Rationale and indications for perfusion in liver tumors: current data. World J Surg. 1987;11:534–40.
Ramadori G, Cameron S. Effects of systemic chemotherapy on the liver. Ann Hepatol. 2010;9:133–43.
Kemeny N, Daly J, Oderman P, Geller N, Botet J, Oderman P, et al. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med. 1987;107:459-465.
Kemeny N, Gonen M, Sullivan D, Schwartz L, Benedetti F, Saltz L, et al. Phase I study of hepatic arterial infusion of floxuridine and dexamethasone with systemic irinotecan for unresectable hepatic metastases from colorectal cancer. J Clin Oncol. 2001;19:2687–95.
Kemeny N, Jarnagin W, Paty P, Gonen M, Schwartz L, Morse M, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol. 2005;23:4888–96.
Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465–71.
Shitara K, Munakata M, Kudo T, Kasai M, Muto O, Okada R, et al. [Combination chemotherapy with hepatic arterial infusion of 5-fluorouracil (5-FU) and systemic irinotecan (CPT-11) in patients with unresectable liver metastases from colorectal cancer.] Gan To Kagaku Ryoho. 2006;33:2033–7.
Gallagher DJ, Capanu M, Raggio G, Kemeny N. Hepatic arterial infusion plus systemic irinotecan in patients with unresectable hepatic metastases from colorectal cancer previously treated with systemic oxaliplatin: a retrospective analysis. Ann Oncol. 2007;18:1995–9.
Lise M, Pilati P, Da Pian P, Mocellin S, Ori C, Casara D, et al. Hyperthermic isolated liver perfusion for unresectable liver cancers: pilot study. J Chemother. 2004;16(Suppl 5):37–9.
Grover AC, Libutti SK, Pingpank JF, Helsabeck C, Beresnev T, Alexander HR Jr. Isolated hepatic perfusion for the treatment of patients with advanced liver metastases from pancreatic and gastrointestinal neuroendocrine neoplasms. Surgery. 2004;136:1176–82.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47.
Wang C, Li J. An update on chemotherapy of colorectal liver metastases. World J Gastroenterol. 2012;18:25–33.
Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–9.
Rougier P, Milan C, Lazorthes F, Fourtanier G, Partensky C, Baumel H, et al. Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer. Br J Surg. 1995;82:1397–400.
Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–66.
Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–22.
Power DG, Kemeny NE. Chemotherapy for the conversion of unresectable colorectal cancer liver metastases to resection. Crit Rev Oncol Hematol. 2011;79:251–64.
Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil–leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147.
Acknowledgment
This project used the UPCI Clinical Pharmacology Analytical Facility (CPAF) and was supported in part by award NIH R21 U01CA099168, Koch Regional Perfusion Center and CCSG Grant P30CA047904.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magge, D., Zureikat, A.H., Bartlett, D.L. et al. A Phase I Trial of Isolated Hepatic Perfusion (IHP) Using 5-FU and Oxaliplatin in Patients with Unresectable Isolated Liver Metastases from Colorectal Cancer. Ann Surg Oncol 20, 2180–2187 (2013). https://doi.org/10.1245/s10434-013-2960-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-2960-3