ABSTRACT
Background
Locoregional recurrence significantly impacts survival and quality of life in patients with ovarian carcinoma. We hypothesize that local administration of paclitaxel-loaded expansile nanoparticles (pax-eNP) at the time of cytoreductive surgery decreases local tumor recurrence.
Methods
In vitro cytotoxicity of pax-eNP was assessed against both the OVCAR-3 human ovarian cancer cell line and tumor cells isolated from a malignant pleural effusion from a patient with multidrug-resistant ovarian cancer. A murine xenogenic model involving surgical cytoreduction of established OVCAR-3 intra-abdominal tumor was used to evaluate in vivo efficacy of intraoperative intraperitoneal (IP) injection of 10 mg/kg of paclitaxel either as pax-eNP or paclitaxel in Cremophor EL/ethanol solution (pax-C/E) versus empty eNP controls. Cytoreductive surgery and intraoperative treatment were performed 4 weeks after established tumor. All animals were sacrificed when empty eNP controls displayed extensive evidence of disease progression.
Results
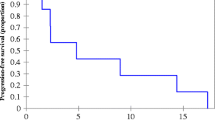
Labeled-eNP entered tumor cells in vitro within 4 h and specifically accumulated at sites of tumor in vivo. Pax-eNP exhibited dose-dependent cytotoxicity in both OVCAR-3 and patient tumor cells isolated from a malignant pleural effusion and effectively prevented tumor recurrence following debulking (p = 0.003 vs. empty eNP). Furthermore, pax-eNP-treated animals did not develop severe recurrent carcinomatosis compared with 43 % of the pax-C/E-treated cohort, suggesting that single-dose intracavitary pax-eNP is more effective than an equivalent dose of pax-C/E.
Conclusions
Expansile nanoparticles readily enter human ovarian tumor cells and localize to sites of tumor in vivo with pax-eNP cytotoxicity resulting in superior inhibition of locoregional tumor recurrence following cytoreductive surgery.





Similar content being viewed by others
REFERENCES
National Cancer Institute. NCI clinical announcement on intraperitoneal chemotherapy in ovarian cancer. 2006. http://ctep.cancer.gov/highlights/ovarian.html. Accessed 5 Jan 2006.
Lewin S, Herzog T, Barrena Medel N, et al. Comparative performance of the new versus old FIGO staging system for endometrial cancer. Gynecol Oncol. 2010;116:S6–7.
Ang C, Chan KKL, Bryant A, et al. Ultra-radical (extensive) surgery versus standard surgery for the primary cytoreduction of advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;(4):CD007697. doi:10.1002/14651858.CD007697.pub2.
Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2008. Bethesda: National Cancer Institute; 2011.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66.
du Bois A, Quinn M, Thigpen T, et al. 2004 Consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004). Ann Oncol. 2005;16 (Suppl 8):viii7–12.
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43.
Walker J, Armstrong DK, Huang HK, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;100(1):27–32.
Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21 (17):3194–200.
Kirmani S, Braly PS, McClay EF, et al. A comparison of intravenous versus intraperitoneal chemotherapy for the initial treatment of ovarian cancer. Gynecol Oncol. 1994;54 (3):338–44.
Coleman, Brady, McMeekin, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;122 (1):111–5.
Werner ME, Karve S, Sukumar R, et al. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32 (33):8548–54.
Sloat BR, Sandoval MA, Li D, et al. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int J Pharm. 2011;409 (1–2):278–88.
Sawicki JA, Anderson DG, Langer R. Nanoparticle delivery of suicide DNA for epithelial ovarian cancer therapy. Adv Exp Med Biol. 2008;622:209–19.
Schulz MD, Zubris KA, Wade JE, et al. Paclitaxel-loaded expansile nanoparticles in a multimodal treatment model of malignant mesothelioma. Ann Thorac Surg. 2011;92 (6):2007–13.
Griset AP, Walpole J, Liu R, et al. Expansile nanoparticles: synthesis, characterization, and in vivo efficacy of an acid responsive polymeric drug delivery system. J Am Chem Soc. 2009;131:2469–71.
Hamilton TC, Young RC, Louie KG, et al. Characterization of a xenograft model of human ovarian carcinoma which produces ascites and intraabdominal carcinomatosis in mice. Cancer Res. 1984;44:5286–90.
Massazza G, Tomasoni A, Lucchini V, et al. Intraperitoneal and subcutaneous xenografts of human ovarian carcinoma in nude mice and their potential in experimental therapy. Int J Cancer. 1989;44:494–500.
Joerger M, et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European Organization for Research and Treatment of Cancer-Pharmacology and Molecular Mechanisms Group and New Drug Development Group. Clin Cancer Res. 2007;13:6410.
Yen MS, Juang CM, Lai CR, et al. Intraperitoneal cisplatin-based chemotherapy vs. intravenous cisplatin-based chemotherapy for stage III optimally cytoreduced epithelial ovarian cancer. Int J Gynaecol Obstet. 2001;72 (1):55–60.
Konner JA, Grabon D, Pezzulli S, et al. A phase II study of intravenous (IV) and intraperitoneal (IP) paclitaxel, IP cisplatin, and IV bevacizumab as first-line chemotherapy for optimal stage II or III ovarian, primary peritoneal, and fallopian tube cancer [abstract]. J Clin Oncol. 2009;27:5539.
Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170 (4):974–9.
Hoskins WJ, Bundy BN, Thigpen JT, et al. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47 (2):159–66.
Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20 (5):1248–59.
Araujo L, Lobenberg R, Kreuter J. Influence of the surfactant concentration on the body distribution of nanoparticles. J Drug Targeting. 1999;6:373–85.
Armstrong DK, Fleming GF, Markman M, Bailey HH. A phase I trial of intraperitoneal sustained-release paclitaxel microspheres (Paclimer) in recurrent ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103:391–6.
Zubris KA, Colson YL, Grinstaff MW. Hydrogels as intracellular depots for drug delivery. Mol Pharm. 2012;9(1):196–200.
Lammers T, Hannink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99(3):392–7.
Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.
Kim BY, Rutka JT, Chan WC, et al. Nanomedicine. N Engl J Med. 2010;363:2434–43.
Gilmore D, Colson YL. Tumor targeted nanoparticles: a modern day Trojan horse. Semin Thorac Cardiovasc Surg. 2011;23(1):10–11.
Yang CY, Liaw YF, Chu CM, Sheen IS. White count, pH and lactate in ascites in the diagnosis of spontaneous bacterial peritonitis. Hepatology. 1985;5(1):85–90.
Emoto S, Kitayama J, Yamaguchi H, Ishigami H, Kaisaki S, Nagawa H. Analysis of pO2 in malignant ascites of patients with peritoneal dissemination of gastric cancer. Case Rep Oncol. 2010;3:344–8.
Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2:343–66.
Boyer MJ, Tannock IF. Regulation of intracellular pH in tumor cell lines: influence of microenvironmental conditions. Cancer Res. 1992;52:4441–7.
ACKNOWLEDGMENT
The authors express their appreciation to Brigham and Women’s Hospital, the Dana-Farber Cancer Institute Animal Facility, and Beth Israel Deaconess Medical Center Confocal Imaging Core who kindly provided their expertise and guidance. This work was supported by the Center for Integration of Medicine and Innovative Technology, the Cross-Disciplinary Training in Nanotechnology for Cancer, NIH R25 CA153955, and the NSF DMR-1006601.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gilmore, D., Schulz, M., Liu, R. et al. Cytoreductive Surgery and Intraoperative Administration of Paclitaxel-loaded Expansile Nanoparticles Delay Tumor Recurrence in Ovarian Carcinoma. Ann Surg Oncol 20, 1684–1693 (2013). https://doi.org/10.1245/s10434-012-2696-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2696-5