Abstract
Purpose
This study was designed to evaluate the benefit of staging laparoscopy (SL) in patients with suspected hilar cholangiocarcinoma (HCCA) during the past 10 years. Only 50–60% of patients with HCCA who undergo laparotomy are ultimately amenable to a potentially curative resection. In a previous study, we recommended routine use of SL to prevent unnecessary laparotomies. The accuracy of imaging techniques, however, has significantly improved during the past decade, which is likely to impact the yield and accuracy of SL.
Methods
From 2000 to 2010, 195 patients with suspected HCCA were analyzed. The yield and accuracy of SL were calculated by dividing total number of avoided laparotomies by the total number of laparoscopies or by all patients with unresectable disease, respectively. Factors associated with better yield and accuracy were assessed.
Results
Of 195 patients with HCCA, 175 underwent SL. The yield of SL was 14% and the accuracy was 32%. Operative morbidity of SL was 3%, and operative morbidity of laparotomy for unresectable disease was 33%. No clear factors that influenced the yield of SL were found.
Conclusion
Overall yield and accuracy of SL for HCCA in the present series decreased to 14% and 32%, respectively, compared with earlier reports. This finding is likely the result of improved imaging techniques that evolved during the past decade. The place of SL in the workup of patients with HCCA needs to be reconsidered, and one should decide whether the declining additional value of SL still outweighs the drawbacks of SL.
Similar content being viewed by others
Hilar cholangiocarcinoma (HCCA), or Klatskin tumor, is a rare cancer that arises at the confluence of the right and left hepatic ducts, with an incidence of 2.5 per 100,000. Radical surgery is the only curative treatment due to the lack of effective systemic therapy. Many different imaging techniques, including computed tomography (CT), abdominal ultrasound (US), percutaneous transhepatic cholangiography (PTC), endoscopic retrograde cholangiopancreatography (ERCP), positron emission tomography (PET), and magnetic resonance imaging (MRI), are used for the staging of HCCA. Despite the multitude of diagnostic tools, only 50–60% of patients who are surgically explored are ultimately amenable to a potentially curative resection.1
Peritoneal, nodal, and liver metastases, as well as locally advanced disease, are the main reasons for unresectability in patients with HCCA. In an attempt to prevent an unnecessary laparotomy, staging laparoscopy (SL) has been performed in patients with HCCA, thereby decreasing operative morbidity and length of hospital stay associated with a laparotomy.2–5 In 2002, we evaluated the use of SL in patients with malignant proximal bile duct obstruction in our center and concluded, on the basis of a yield of 42%, that SL should be performed routinely in the workup for these patients.4
The accuracy of radiologic imaging techniques has continued to improve. Recently, additional techniques, such as PET and multi-slice CT, have been added to the staging armamentarium. These advances in staging are likely to impact the yield and accuracy of SL. Hence, the purpose of this study was to evaluate the benefit of SL for staging of patients with suspected hilar cholangiocarcinoma during the past 10 years in our institution, in which time imaging modalities have greatly improved.
Materials and Methods
All patients presenting with a suspicion on hilar cholangiocarcinoma between May 2000 and May 2010 were retrieved from a prospectively collected database. Patients with distal, intrahepatic, and gallbladder cholangiocarcinoma were excluded from this analysis. All patients had histological confirmation of their disease.
Staging and Laparoscopy
Patients underwent extensive preoperative imaging, either performed in the referring hospital or in our own center. Standard staging examinations included transabdominal ultrasound with Doppler imaging of the blood vessels at the liver hilum, four-phase CT, biliary imaging, and drainage, by ERCP or PTC. All images from referring hospitals were revaluated by our own radiologists, who are experienced in biliary tract morphology. MRI was used selectively, and PET-CT was performed in most patients during the past 3 years of the study. After imaging, cases were reviewed in a multidisciplinary, hepatobiliary meeting and staged according to the modified Bismuth–Corlette classification based on all available imaging studies.6 In addition, other criteria for resectability were taken into account, such as extrahepatic disease and vascular involvement. Laparoscopy was planned, when feasible, in all patients with HCCA Bismuth types 3 and 4 considered potentially resectable. In patients with HCCA Bismuth types 1 and 2, staging laparoscopy was performed selectively based on surgeon’s preference.
SL was performed under general anesthesia using three trocars at umbilical and left and right subcostal position, as described previously.7,8 The hepatic surface and peritoneal cavity were searched for metastases. The hepatoduodenal ligament and gallbladder were inspected for the presence of local tumor mass or enlarged lymph nodes. The lesser omentum was opened and the common hepatic artery inspected along its course, in search of suspicious lymph nodes. Biopsies were undertaken under direct vision using biopsy forceps. Nodal sampling was not performed routinely, unless a lymph node metastasis was suspected. Suspected lesions were biopsied and microscopically analyzed by a pathologist on HE sections. When no metastases were found, patients were planned for resection. Laparoscopic ultrasound was only performed in four patients in this series and therefore not further analyzed.
Laparotomy and Resection
After laparotomy, the abdomen was inspected for peritoneal seeding, distant metastases, or lymph node involvement outside the liver hilum. Suspicious lesions were excised or biopsied and analyzed by frozen-section histology. Tumors were considered unresectable when the following signs were found: peritoneal metastases, hepatic metastases, nodal metastases outside the hepatoduodenal ligament, or locally advanced disease with tumor infiltrating high up into the segmental bile ducts of both hemilivers.9 If more causes of unresectability were found, the one discovered first was reported. More specifically, peritoneal metastases, liver metastases, positive lymph nodes, and locally advanced disease were reported in decreasing order. In case of metastasis, no further surgical intervention was performed. In few selected cases diagnosed as Bismuth type I and II tumors with locally advanced disease, a palliative local hilar resection was performed. In patients with unresectable disease, biliary drainage was accomplished by definitive internal stenting by PTC or ERCP using metal expandable stents, usually within the same hospital admission.10,11
The Modified Clavien-Dindo system was used to grade complications; grades I and II represented minor and grades III, IV, and V represented major complications.12
Statistical Analysis
Patient demographics, preoperative imaging, preoperative Bismuth classification, surgical findings, resectability, and length of hospital stay were analyzed. The yield of laparoscopy was calculated by dividing the number of avoided laparotomies by the total number of laparoscopies. Accuracy was determined by dividing the number of avoided laparotomies by all patients with unresectable disease. Possible factors correlating with accuracy and yield were verified by using a chi-square test or Fishers exact test when numbers in a category were low. The association of different time periods was examined using a test for trend across groups. Statistical analysis was performed by using SPSS version 16.0 software (SPSS Inc. Chicago, IL). P values < 0.05 were considered statistically significant.
Results
Patient Demographics and Preoperative Imaging
A total of 195 patients were considered resectable based on imaging and were subsequently surgically treated in our center. Twenty patients underwent immediate laparotomy without a SL. Fourteen of these patients had Bismuth type 1 or 2 tumors. In the six other patients, the reason for omitting laparoscopy was predominantly a doubtful diagnosis of malignancy.
A total of 175 patients underwent a staging laparoscopy and are analyzed in this study. In ten patients considered resectable based on SL, no laparotomy was performed because of intercurrent medical unfitness or unresectability on subsequent imaging. The median age of patients was 64 (range, 32–79) years, and the male-to-female ratio was 126:69 (65%).
Preoperative staging was extensive and included a CT, abdominal US with Doppler, and direct cholangiography by PTC or ERCP, in nearly all cases (Table 1). SL was performed by an attending surgeon in 108 patients (62%), and by a fellow or senior resident in 87 patients (38%). The median time between SL and laparotomy was 37 (range, 14–90) days.
Surgical Findings
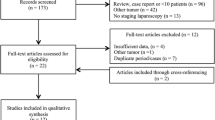
Staging laparoscopy was successfully performed in all patients. Laparoscopic and ultimate findings are shown in Fig. 1. Of all patients who underwent SL, 24 were found unresectable, resulting in an overall yield of 14% (95% confidence interval (CI), 9–20). Patients (n = 10) who did not undergo verification by laparotomy were not included in this calculation. Of all patients who were unresectable (n = 76), 24 were identified by SL, resulting in an accuracy of 32% (95% CI, 18–40). Laparoscopy correctly identified 59% (22/37) of patients with metastases (peritoneal or hepatic) but was hardly able to detect locally advanced disease (2/17) or nodal metastases precluding a curative resection (0/15). Final pathology showed benign disease in 12 patients: chronic fibrosing cholangitis (n = 7), intraductal papillary mucinous neoplasm (n = 8), primary sclerosing cholangitis (n = 1), chronic inflammation (n = 1), and IgG4-associated sclerosing cholangitis (n = 1). A laparoscopic biopsy of suspicious lesions was performed in 96 patients (58%). Based on these findings, an unnecessary laparotomy was avoided in 24 patients. A laparoscopic biopsy was performed in 15 patients who were found unresectable at laparotomy. In six of these patients, potentially detectable metastases (peritoneal or hepatic) were present during laparotomy. The findings of laparoscopic biopsies result in a sensitivity, specificity, negative predictive value, and positive predictive value of 79, 100, 100, and 92%, respectively.
Minor complications (grade I and II) occurred in five patients (3%), and included fever that required antibiotics (n = 2), pain that required prolonged hospital stay (n = 1), cardiac ischemia without elevation of cardiac enzymes (n = 1), and pneumonia (n = 1). Complications were likely to be related to the laparoscopic procedure, only in the patients with pain, pneumonia, and cardiac ischemia. No mortality occurred after laparoscopy. Complications after laparotomy occurred in 17 patients (29%) with unresectable disease and included two major complications (>grade IIIa): wound dehiscence (n = 1), and abdominal sepsis and death after a palliative gastrojejunostomy (n = 1).
The median hospital stay for laparoscopy and laparotomy for patients with unresectable disease was 3 (range, 2–16) days and 14 (range, 4–86) days, respectively (P < 0.001). The time required until definitive internal biliary drainage also was included in the total hospital stay for unresectable patients.
Influencing Factors
Specific subgroups were further analyzed to identify factors that influenced the yield and accuracy of SL. The yield and accuracy of SL in the total group were 14 and 32%, respectively. The yield of SL during the time period 2000–2002 was 19% and seemed to decline to 16% in 2003–2006 and 7% in 2007–2010 (P = 0.13). The accuracy of the three time periods was 39, 30, and 18%, respectively (P = 0.38). The yield and accuracy of different subgroups are shown in Table 2. Only preoperative imaging, including PET-CT, showed a statistically significant decline in yield.
Missed Metastases and Positive Lymph Nodes
We further analyzed the group of patients with a negative laparoscopy who were found unresectable during laparotomy. This group included patients with “missed metastases” (n = 15), positive lymph nodes (n = 21) beyond the hepatoduodenal ligament, and locally advanced disease (n = 19). Of the metastases (hepatic and peritoneal), six were suspicious during SL and were consequently biopsied. Due to false-negative histological results, probably as a result of inadequate sampling, these patients underwent a laparotomy. Of all metastases, nine were >0.5 cm, suggesting that these could have been detected by SL (Table 3). Most of the positive lymph nodes (n = 10) were encountered around the common hepatic artery (Table 4). Twenty-one positive lymph nodes were encountered, of which 13 could be potentially reached by SL.
Discussion
The present study has shown that the accuracy of SL for suspected HCCA during the past decade in our department was 32% and that the overall yield was 14%. In other words, of all patients with HCCA who underwent a staging laparoscopy, 14% was diagnosed with advanced disease and could be spared an unnecessary laparotomy. Of all HCCA patients with unresectable disease, 32% was detected by laparoscopy.
The yield and accuracy of staging laparoscopy depend on many different factors, including the a priori chance of unresectable disease, the likelihood of finding unresectability, and the skills and determination of the surgeon performing SL. The goal of staging laparoscopy is, in particular, to exclude peritoneal metastases and small liver metastases, for which other noninvasive tests lack accuracy. Nonetheless, the overall yield and accuracy of staging laparoscopy are calculated according to the proportion of unresectable patients regardless of the cause of unresectability. Hence, the yield of laparoscopy directly depends on the proportion of patients with unresectable disease detectable by laparoscopy, namely the patients with peritoneal or hepatic metastases. This is important to keep in mind, because during the past years the imaging of HCCA patients has much improved. Accordingly, patients with HCCA considered for treatment are better staged, and consequently, a higher rate of patients is diagnosed with unresectable disease by imaging without the need for laparoscopy. Therefore, the patients who are ultimately selected for laparoscopy are less likely to have metastases and, in addition, have smaller metastases, which are less likely to be detected by imaging or laparoscopy.
The yield and accuracy of staging laparoscopy in patients with HCCA found in the current study are considerably lower than those reported in previous studies (Table 5), including the study performed in our own institution, which spanned the period 1992–2000. The most likely explanations for these differences, as mentioned earlier, include the better selection of patients for laparoscopy, the impact of improved imaging, and the decreased proportion of potentially detectable lesions by laparoscopy (hepatic or peritoneal metastases). Furthermore, although HCCA is not known for its aggressive course and rapid development of metastases, the time between SL and definitive laparotomy may have influenced results, because metastases could have developed in between the two procedures.
The latest study to address the benefit of laparoscopy in HCCA included patients until 2004;3 the other studies in the literature are even less timely.2,4,5,9 These time differences are important considering the impact of improved imaging during past years. This is reflected by the proportion of HCCA patients undergoing laparoscopy who were ultimately resected. In the present study, 51% of all HCCA patients undergoing laparoscopy underwent resection, as opposed to 27–45% reported in previous studies. The proportion of metastases in the present study was 22% (31/151), which is substantially lower than in previous studies (29–33%). These factors are likely to have influenced the yield and accuracy found in this study. Last, 12 patients showed benign disease at final pathology, and because these patients obviously had no metastases, this also influenced the yield.
The discrepancy with the results of our previous study is striking, for which two additional reasons can be held responsible. First, during the first period, SL was combined with a laparoscopic ultrasound examination as part of an ongoing study evaluating the benefit of SL and laparoscopic ultrasound.13 As a consequence, more time was spent on SL during the first period, which also was more extensive and performed with more dedication. Because the previous study did not show any added value of laparoscopic ultrasound, this procedure was abandoned during SL in the subsequent series.4 Second, during the first period there was uncertainty about the origin of the carcinoma—gallbladder or hilar—in 20% of patients. During this period, these patients underwent SL, in which a gallbladder carcinoma was finally diagnosed. Patients with gallbladder cancer do have a high likelihood of metastatic disease, and therefore the inclusion of this subset of patients has a considerable impact on the amount of metastases found by SL.5,14 During the past period, we were able to make a more accurate diagnosis based on preoperative, cross-sectional imaging and only included patients with a high suspicion of HCCA.
We could only identify a statistically significant decline in the yield of patients preoperatively staged with PET-CT (Table 2). PET-CT is primarily performed to exclude metastases, which also is the primary goal of SL, thus one could expect this finding. Kim et al. found metastases in 7% of patients with the addition of PET-CT to the staging protocol.15 Statistical significance directly depends on the number of events in the two groups compared. The number of events (total positive laparoscopies) in the total group was only 24 (14%). Hence, the current study, although by far the largest to date, is probably underpowered to draw valid conclusions regarding specific factors determining yield and accuracy. Nonetheless, the yield and accuracy of SL probably declined over time in our series, and PET-CT and associated better staging might have further decreased the added value of SL in HCCA patients. However, it should be borne in mind that PET-CT was only performed during the last period, therefore, these factors cannot be analyzed independently. PET-CT also was not performed in all patients during the last period, which introduces a possible selection bias.
Although the yield and accuracy of SL were not clearly higher when performed by an attending surgeon compared with a senior resident or fellow, we believe that SL should be performed by an experienced surgeon. We identified several unresectable patients who could have been spared a laparotomy with a more extensive SL. Twenty-one patients were identified with distant positive lymph nodes precluding a curative resection at laparotomy. We believe that there is room for improvement and that with extensive SL with more attention for lymph nodes, an additional 13 patients (with positive nodes nearby the celiac trunk or common hepatic artery) could have been identified with positive lymph nodes during SL, improving the yield of SL to 20% (Tables 4 and 5). Hence, the use of laparoscopic ultrasound would have possibly increased yield and accuracy of lymph node assessment.
Finally, the benefit of avoiding an unnecessary laparotomy with the associated morbidity and related increase in hospital stay of 9 days in unresectable patients should be weighed against the drawbacks of SL, namely morbidity and hospital stay of SL, disadvantages of waiting time between SL and laparotomy, operating time, and healthcare costs. This study questions the conclusion of previous studies, including our own published in 2002, that patients with HCCA should undergo SL routinely.2–5,9 In the light of our recent results, we have reconsidered the place of SL in our workup of patients with HCCA. Several options may be considered, including:
-
1)
A more selective approach to submitting patients with HCCA for SL, including only patients with Bismuth type 3 and 4 tumors, and patients with suspicion on metastases;
-
2)
The use of SL as a short procedure preceding laparotomy in one session. This setup requires flexibility of the operation schedule while one needs to be prepared to convert to laparotomy and an extensive resection when SL proves negative;
-
3)
Application of SL as a protocol performed by dedicated (staff) surgeons experienced in laparoscopic procedures. A standard checklist will improve the accuracy of SL and call for detail in the sampling of distant lymph nodes.
In conclusion, accurate staging of patients with HCCA remains difficult. SL avoided an unnecessary laparotomy in 14% of patients after complete imaging, with an accuracy of 32%. It is likely that these numbers will even further decline with the use of more sophisticated imaging techniques. In contrast to previous reports, the results of the present study do not justify the routine use of SL in patients with resectable HCCA. The place of SL in the workup of patients with HCCA needs to be reconsidered, and one should decide whether the declining additional value of SL outweighs the drawbacks of SL.
References
Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250(2):210–8.
Connor S, Barron E, Wigmore SJ, Madhavan KK, Parks RW, Garden OJ. The utility of laparoscopic assessment in the preoperative staging of suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2005;9(4):476–80.
Goere D, Wagholikar GD, Pessaux P, Carrere N, Sibert A, Vilgrain V, et al. Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc. 2006;20(5):721–5.
Tilleman EH, de Castro SM, Busch OR, Bemelman WA, van Gulik TM, Obertop H, et al. Diagnostic laparoscopy and laparoscopic ultrasound for staging of patients with malignant proximal bile duct obstruction. J Gastrointest Surg. 2002;6(3):426–30.
Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235(3):392–9
Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31–8.
Bemelman WA, van Delden OM, van Lanschot JJ, de Wit LT, Smits NJ, Fockens P, et al. Laparoscopy and laparoscopic ultrasonography in staging of carcinoma of the esophagus and gastric cardia. J Am Coll Surg. 1995;181(5):421–5.
Bemelman WA, de Wit LT, van Delden OM, Smits NJ, Obertop H, Rauws EJ, et al. Diagnostic laparoscopy combined with laparoscopic ultrasonography in staging of cancer of the pancreatic head region. Br J Surg. 1995;82(6):820–4.
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–17.
Cheng JL, Bruno MJ, Bergman JJ, Rauws EA, Tytgat GN, Huibregtse K. Endoscopic palliation of patients with biliary obstruction caused by nonresectable hilar cholangiocarcinoma: efficacy of self-expandable metallic Wallstents. Gastrointest Endosc. 2002;56(1):33–9.
Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340(8834–8835):1488–92.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-13.
Nieveen van Dijkum EJ, de Wit LT, van Delden OM, Kruyt PM, van Lanschot JJ, Rauws EA, et al. Staging laparoscopy and laparoscopic ultrasonography in more than 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg. 1999;189(5):459–65.
Vollmer CM, Drebin JA, Middleton WD, Teefey SA, Linehan DC, Soper NJ, et al. Utility of staging laparoscopy in subsets of peripancreatic and biliary malignancies. Ann Surg. 2002;235(1):1–7.
Kim JY, Kim MH, Lee TY, Hwang CY, Kim JS, Yun SC, et al. Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol. 2008;103(5):1145–51.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ruys, A.T., Busch, O.R., Gouma, D.J. et al. Staging Laparoscopy for Hilar Cholangiocarcinoma: Is it Still Worthwhile?. Ann Surg Oncol 18, 2647–2653 (2011). https://doi.org/10.1245/s10434-011-1576-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1576-8