Abstract
Background
Limited data are available on the use of cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion (HIPEC) in patients with recurrent stage III ovarian cancer.
Methods
Patients with recurrent, heavily pretreated ovarian cancer were enrolled onto a phase II multimodal protocol consisting of extensive cytoreduction followed by HIPEC.
Results
Forty-two women were treated from October 2002 until January 2009. Chemoperfusion was performed with cisplatin in 59% and oxaliplatin in 41% of patients. A macroscopically complete resection was achieved in 50% of patients. No mortality occurred, and the major morbidity rate was 21%. After a mean follow-up of 21 months, median overall survival (OS) was 37 months (95% confidence interval 12.2–61.8) and median progression-free survival was 13 months (95% confidence interval 6.9–19.1). In univariate analysis, OS was influenced by completeness of cytoreduction, type of chemoperfusion drug, nodal status, and tumor grade. In a Cox regression model, only completeness of cytoreduction (hazard ratio 0.06–0.8, P = .022) and tumor grade (hazard ratio 1.23–12.6, P = .021) were independent predictors of OS.
Conclusions
In selected patients with heavily pretreated recurrent ovarian cancer, cytoreduction combined with HIPEC may provide a meaningful OS with acceptable morbidity. Optimal results are achieved in patients with a macroscopically complete resection and biologically favorable disease.
Similar content being viewed by others
With >20,000 new cases annually in the United States and 40,000 in Europe, ovarian cancer (OC) represents the second most common gynecological malignancy.1,2 Almost 70% of patients are diagnosed with stage IIIC disease, and only a modest improvement in survival has been achieved over the last two decades.3 Even after optimal cytoreduction followed by adjuvant chemotherapy, most patients with stage III disease will develop a recurrence.4
The utility of a secondary cytoreduction in patients with recurrent disease remains a matter of debate. A recent meta-analysis of cohort studies of patients who underwent cytoreduction for recurrent OC demonstrated a median survival of >30 months.5 The only statistically significant predictor of postrecurrence survival was the proportion of patients undergoing a macroscopically complete cytoreduction. The results of the DESKTOP I multicenter trial suggest that the presence of peritoneal carcinomatosis does not adversely affect 2-year survival when a complete resection can be performed.6 Many patients with a macroscopically complete resection will harbor minimal residual disease, and therefore theoretically might benefit from the addition of intraperitoneal (IP) chemotherapy as an adjunct to surgery. Several large randomized trials have demonstrated a statistically significant survival benefit associated with IP platinum-based chemotherapy as a first-line treatment after primary cytoreduction.7–9
Since its original description by Spratt in 1980, hyperthermic intraperitoneal chemotherapy (HIPEC) is increasingly used as an adjunct to surgery in patients with peritoneal surface malignancy.10 The rationale for hyperthermic drug administration is based mainly on thermal enhancement of the cytotoxicity of many cytostatic drugs, including the alkylating agents and the platinum compounds.11 Numerous phase II trials of HIPEC in primary advanced or recurrent OC were reported that used a variety of chemotherapeutic regimens and delivery methods; only two trials reported on > 50 patients.12,13 A systematic review of the available early-phase data suggested promising overall survival (OS) outcomes (range 22–54 months); the associated morbidity and mortality (5–36% and 3%, respectively) are in keeping with that of other major abdominal procedures.14
The objective of this phase II study was to assess the safety and efficacy of extensive cytoreduction and HIPEC in a cohort of patients with heavily pretreated recurrent OC.
Patients and Methods
Study Design
This was a prospective single-institution phase II trial that aimed to investigate the safety and efficacy of combined cytoreduction and HIPEC in patients with heavily pretreated recurrent OC. The end points were treatment related morbidity and mortality, progression-free survival, and OS. The treatment protocol was approved by the Ghent University Hospital local ethical committee.
Patient Selection
Eligible patients presented with potentially resectable heavily pretreated recurrent OC. Inclusion criteria were age <75 years, Eastern Cooperative Oncology Group score ≤2, no major cardiac or respiratory disease, and normal renal and liver function. The decision to offer surgery was made by a multidisciplinary team including a surgeon, medical oncologist, and radiation oncologist. Clinical variables regarded as favoring surgery were an interval between the primary surgery and a recurrence of at least 6 months, absence of clinical ascites, and good performance status. Preoperative workup included computed tomographic scan of the abdomen and chest, and positron emission tomography to exclude systemic metastases. Written informed consent was obtained before surgery.
Surgery and Intraoperative Chemotherapy
All patients underwent extensive cytoreduction including peritonectomy of the involved mesothelial surfaces. Care was taken to avoid the use of energy sources on the small bowel surfaces. Small bowel anastomoses were performed before hyperthermic chemoperfusion because a varying degree of small bowel edema usually develops after chemoperfusion. Lymphadenectomy was performed only of nodes that were deemed clinically or radiographically suspicious. The extent of disease before cytoreduction was scored according to a simplified scale ranging from 1 to 7 indicating the number of abdominal regions affected.15 The completeness of cytoreduction (CC) was scored as follows: 0, removal of all visible tumor; 1, minimal residual disease (e.g., miliary implants on small bowel surfaces or thin layer of malignant tissue on the liver capsule treated with argon fulguration); and 2, more extensive residual disease.
Depending on assumed platinum sensitivity, HIPEC (target temperature 40.5–41°C) was performed with either cisplatin (dose range 100–250 mg/m2 in peritoneal dialysis solution according to creatinine clearance) or oxaliplatin (460 mg/m2 in dextrose 5%). Oxaliplatin has demonstrated activity in recurrent platinum-resistant OC and was preferred in patients with presumed cisplatin resistance or documented hypersensitivity.16 IP chemoperfusion with oxaliplatin (duration, 30 min) was performed by means of an open abdomen (coliseum) method.17 The skin was sutured to a retractor frame positioned above the patient’s abdomen. This retractor frame was covered with a plastic hood allowing aspiration and evacuation of vapor escaping from the abdominal cavity. When cisplatin is provided, chemoperfusion was performed during 90 min after temporary skin closure. The shorter duration of chemoperfusion with oxaliplatin is based on a more rapid systemic absorption and a more pronounced locoregional toxicity with longer chemoperfusion times.18 The perfusion circuit always consisted of two Tenckhoff-type inflow drains; three multiperforated outflow drains positioned in the pelvis, right upper abdomen, and left upper abdomen; a heat exchanger element; and a peristaltic roller pump. Abdominal and central temperatures were monitored by three thermocouple probes and an esophageal temperature probe, respectively. Controlled hypothermia (34°C) was maintained before the start of chemoperfusion. Systemic sodium thiosulfate (4 g/m2 during the chemoperfusion and 12 g/m2 in the first 6 h after chemoperfusion) was administered intravenously to patients who underwent chemoperfusion with cisplatin. All procedures were performed by an experienced surgical oncologist (W.P.C.).
Follow-Up
Patients were followed by clinical assessment, assessment of cancer antigen 125, and computed tomographic scan of the abdomen every 3 months. Adjuvant chemotherapy was initiated in fit patients starting 6–8 weeks after surgery.
Data Collected
Variables analyzed included demographic data, surgical parameters, postoperative outcome and length of stay, pathology details, and oncological outcome. Major morbidity was defined as any postoperative complication causing reoperation and/or prolonged length of stay, and minor morbidity was defined as any complication requiring therapy but not resulting in either reoperation or prolonged length of stay.
Statistical Analysis
Data are expressed as median (range) unless indicated otherwise. Actuarial overall and progression-free survival were estimated by the Kaplan–Meier method. The impact of clinical variables on OS time was assessed by the log rank test and Cox multivariate regression. Statistical significance was assumed when the probability of a type I error was ≤.05. All P values are two sided. Calculations and graph drawing were performed with SigmaPlot 11 for Windows (Systat Software, San Jose, CA).
Results
Patient Population
From October 2002 until January 2009, a total of 42 women with recurrent epithelial OC were treated. The clinical and demographic details of the study cohort are summarized in Table 1. All patients had undergone at least one previous major cytoreduction, and more than three quarters were treated with several lines of systemic chemotherapy.
Surgery and HIPEC
Twenty-nine patients (69%) had four or more affected abdominal regions (Table 2). The regions at highest risk of recurrence were the residual omentum, small pelvis, and right subdiaphragmatic space. The heterogeneous behavior of the disease resulted in a wide variety of surgical maneuvers including a low or ultralow anterior resection in almost one in two patients (Table 2). Despite the extent of these procedures, the permanent ostomy rate was limited to 9%. In half of the patients a macroscopically complete resection was achieved (Table 3). In patients with a CC resection classified as 1 (minimal residual disease), thin layers of tumor tissue on the surface of the liver, large vessels, or small bowel serosal surfaces were treated maximally with argon beam electrocoagulation. Seventeen patients (41%) underwent chemoperfusion with oxaliplatin because of cisplatinum resistance or hypersensitivity.
Morbidity and Mortality
No technical problems occurred during the hyperthermic chemoperfusion. In patients who underwent chemoperfusion with oxaliplatin, which is administered in dextrose 5% for reasons of chemical stability, continuous administration of insulin and correction of metabolic acidosis were necessary.19 No postoperative mortality occurred (Table 3). Major morbidity was seen in 21%, and three patients needed reoperation for necrosis of the ureter, staple line bleeding, and thoracic empyema, respectively. The most frequent minor complications were prolonged ileus, urinary tract infection, and wound infection. In patients treated with oxaliplatin, an asymptomatic increase in liver enzymes was noted, as noted previously.20 No other systemic chemotherapy-related toxicity was observed. All but five patients with a complicated postoperative course (12%) received further intravenous chemotherapy.
Histology
The histopathological details are presented in Table 4. In most patients, a serous papillary tumor was found; a mucinous differentiation was noted in 7%. One in three patients had involved lymph nodes.
Survival Analysis
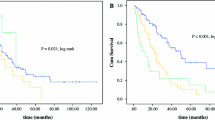
The mean follow-up time was 20.8 months; no patients were lost to follow-up. Median OS was 37 months (95% confidence interval 12.2–61.8). Overall 5-year survival was 41.3% (Fig. 1). Median progression-free survival was 13 months (95% confidence interval 6.9–19.1). Progression-free 5-year survival was 12.5% (Fig. 2).
The results of the univariate time to event analysis are summarized in Table 5. Patient age, time interval from the initial diagnosis, and simplified peritoneal cancer index score were not statistically significantly associated with OS time. Variables with a statistically significant influence on OS time in univariate analysis were nodal status, CC, and tumor grade. Survival was also associated with the type of chemoperfusion drug used, although in univariate analysis the effect failed to reach statistical significance. The four variables with a significant or near-significant P value in univariate analysis were entered as independent variables in a Cox multivariate regression model (Table 6). Variables that independently affected OS were tumor differentiation (hazard ratio 1.23–12.6, P = .021, Fig. 3) and CC (hazard ratio 0.06–0.8, P = .022, Fig. 4). Nodal status and type of chemoperfusion drug did not independently affect OS.
Discussion
Most patients (70–90%) with International Federation of Gynecology and Obstetrics stage III OC will develop a recurrence after cytoreduction and first-line platinum- and paclitaxel-based chemotherapy.
The value of repeat surgery in patients with recurrent disease remains a matter of debate. Several cohort studies support the use of cytoreduction in patients with a disease-free interval of >6 months, limited extent of disease, and good performance status.5,21–23
The results from the multicenter DESKTOP OVAR trial, which included 267 patients with recurrent OC treated surgically, showed a median survival of 45 months in completely resected patients.24 Interestingly, although the presence of peritoneal carcinomatosis represented an adverse prognostic factor, patients in whom the peritoneal disease could be completely resected reaped a similar survival benefit.6 In women with advanced OC, the IP administration of chemotherapy as an adjunct to surgery is based on a solid theoretical rationale, and its efficacy has been demonstrated in large randomized trials.25 Recently, cytoreduction followed by HIPEC has been increasingly used in patients with peritoneal carcinomatosis from low-grade appendiceal or colorectal origin.26,27 Intraoperative hyperthermic chemoperfusion ensures IP delivery of the drug to all peritoneal surfaces at risk and allows exploitation of the synergistic antitumor effect of combined hyperthermia.
Many phase II trials of cytoreduction and HIPEC that used a variety of cytotoxic regimens in advanced primary, relapsing, or recurrent OC have been reported (Table 7).14,28 Our experience confirms the feasibility and safety of extensive cytoreduction followed by HIPEC. Specifically, only mild and manageable locoregional and systemic drug toxicities were noted despite the very high dose regimens. Several technical aspects are important in this regard. First, because the toxicity of hyperthermic chemoperfusion is related to increasing intra-abdominal temperature, we use mild hyperthermia (<41°C) to prevent excessive edema of the small bowel.29 Second, when using oxaliplatin, which has to be administered in dextrose 5%, continuous corrections of glycemia and blood pH are mandatory during the chemoperfusion.
We found, in keeping with other reports, that the outcome in this patient group is a function of CC rather than of the initial tumor load, and that both variables are not necessarily related. Patients may have extensive but easily resectable tumor bulk, whereas others may have many small implants on the small bowel alone that may preclude complete resection.
Our results confirm that oxaliplatin is active in patients with cisplatin-resistant recurrent disease. Although in univariate analysis a trend toward better survival was noted in cisplatin-sensitive patients, cisplatin sensitivity did not convey independent prognostic information in a multivariate model.
A fundamental question in patients with advanced or recurrent OC is whether the natural history of the disease can be influenced by surgical resection. It has been argued that the inherent biology of the disease will predict survival, irrespective of efforts to obtain complete cytoreduction.30 Our results suggest that the presence of a biologically unfavorable (grade 3) tumor has an independent adverse effect on OS (mean 12 vs. 70 months, P < .001). On the other hand, in a multivariate regression model, achieving a complete cytoreduction remained an independent prognosticator. Moreover, cytoreduction may have a beneficial symptomatic effect in many patients with large deposits causing pain or subobstruction. Also, small residual nodules grow more rapidly and will therefore be more sensitive to the effects of adjuvant cytotoxic therapy.31 Any survival benefit gained by extensive surgery should be weighed against the potential for postoperative morbidity and deterioration of the quality of life. In most experienced centers, the morbidity of cytoreduction and HIPEC is comparable to that seen after any major abdominal procedure. A survey has demonstrated a good to excellent quality of life in patients who recovered from cytoreduction and HIPEC for peritoneal carcinomatosis.32
Several questions remain unanswered. First, despite the compelling theoretical rationale to treat peritoneal minimal residual disease with IP hyperthermic chemoperfusion and the results from a growing number of cohort studies, the added value of HIPEC versus surgery alone remains to be formally demonstrated.33 A randomized trial of secondary cytoreduction with or without HIPEC in advanced OC was initiated at the Netherlands Cancer Institute (OVHIPEC trial; ClinicalTrials.gov identifier NCT00426257) and is expected to complete enrollment in 2011. Second, there is as yet no consensus on the optimal chemoperfusion drug, delivery method, IP target temperature, or duration of chemoperfusion. Finally, the role of neoadjuvant and/or complementary intravenous delivery of chemotherapy and/or targeted therapy is at present undefined. An interesting concept in this regard would be to treat patients before HIPEC with targeted therapy to lower interstitial fluid pressure and thereby enhance the tumor penetration of an IP-administered drug.34,35
In conclusion, the results from the present prospective cohort study confirm the safety and feasibility of extensive cytoreduction and HIPEC in patients with heavily pretreated OC. In a multivariate time-dependent regression model, both CC and tumor biology were independent predictors of outcome. A randomized trial comparing cytoreduction alone versus cytoreduction and HIPEC in selected recurrent OC patients is warranted.
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96.
Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–92.
Heintz APM, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. Int J Gynecol Obstet. 2006;95:S161–92.
Leitao MM, Chi DS. Surgical management of recurrent ovarian cancer. Semin Oncol. 2009;36:106–11.
Bristow RE, Puri I, Chi DS. Cytoreductive surgery for recurrent ovarian cancer: a meta-analysis. Gynecol Oncol. 2009;112:265–74.
Harter P, Hahmann M, Lueck HJ, et al. Surgery for recurrent ovarian cancer: role of peritoneal carcinomatosis: exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:1324–30.
Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–5.
Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7.
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43.
Spratt JS, Adcock RA, Muskovin M, Sherrill W, Mckeown J. Clinical delivery system for intra-peritoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–60.
Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–54.
Cotte E, Glehen O, Mohamed F, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for chemoresistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007;31:1813–21.
Ryu KS, Kim JH, Ko HS, et al. Effects of intraperitoneal hyperthermic chemotherapy in ovarian cancer. Gynecol Oncol. 2004;94:325–32.
Bijelic L, Jonson A, Sugarbaker PH. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol. 2007;18:1943–50.
Swellengrebel HAM, Verwaal VJ, Smeenk RM, Antonini N, Zoetmulder FAN. Quantitative intra-operative assessment of peritoneal carcinomatosis—a comparison of three prognostic tools. Eur J Cancer. Suppl 2007;5:258.
Ray-Coquard I, Weber B, Cretin J, et al. Gemcitabine-oxaliplatin combination for ovarian cancer resistant to taxane-platinum treatment: a phase II study from the GINECO group. Br J Cancer. 2009;100:601–7.
Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790–6.
Elias D, Bonnay A, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13:267–72.
De Somer F, Ceelen W, Delanghe J, et al. Severe hyponatremia, hyperglycemia, and hyperlactatemia are associated with intraoperative hyperthermic intraperitoneal chemoperfusion with oxaliplatin. Perit Dial Int. 2008;28:61–6.
Ceelen WP, Peeters M, Houtmeyers P, et al. Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann Surg Oncol. 2008;15:535–41.
Eisenkop SM, Friedman RL, Spirtos NM. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer. 2000;88:144–53.
Salani R, Santillan A, Zahurak ML, et al. Secondary cytoreductive surgery for localized, recurrent epithelial ovarian cancer—analysis of prognostic factors and survival outcome. Cancer. 2007;109:685–91.
Zang RY, Li ZT, Tang J, et al. Secondary cytoreductive surgery for patients with relapsed epithelial ovarian carcinoma: who benefits? Cancer. 2004;100:1152–61.
Harter P, du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702–10.
Ceelen W, Flessner M. Intraperitoneal cytotoxic drug therapy for tumours confined to the peritoneal cavity: biophysical principles and clinical evidence. Nat Rev Clin Oncol. 2009. doi: 10.1038/nrclinonc.2009.217.
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–92.
Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006;7:69–76.
Sugarbaker PH. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for the treatment of advanced primary and recurrent ovarian cancer. Curr Opin Obstet Gynecol. 2009;21:15–24.
Jacquet P, Stephens AD, Averbach AM, et al. Sugarbaker PH. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer. 1996;77:2622–9.
Crawford SC, Vasey PA, Paul J, et al. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005;23:8802–11.
Kohandel M, Sivaloganathan S, Oza A. Mathematical modeling of ovarian cancer treatments: sequencing of surgery and chemotherapy. J Theor Biol. 2006;242:62–8.
Zenasni F, Botella M, Elias D, et al. The long-term impact of hyperthermic intraperitoneal chemotherapy on survivors treated for peritoneal carcinomatosis: a cross-sectional study. Support Care Cancer. 2009;17:1255–61.
Markman M. Hyperthermic intraperitoneal chemotherapy in the management of ovarian cancer: a critical need for an evidence-based evaluation. Gynecol Oncol. 2009;113:4–5.
Matei D, Emerson RE, Schilder J, et al. Imatinib mesylate in combination with docetaxel for the treatment of patients with advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis—a Hoosier Oncology Group trial. Cancer. 2008;113:723–32.
Pietras K, Ostman A, Sjoquist M, et al. Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res. 2001;61:2929–34.
Zanon C, Clara R, Chiappino I, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for recurrent peritoneal carcinomatosis from ovarian cancer. World J Surg. 2004;28:1040–5.
Reichman TW, Cracchiolo B, Sama J, et al. Cytoreductive surgery and intraoperative hyperthermic chemoperfusion for advanced ovarian carcinoma. J Surg Oncol. 2005;90:51–6.
Rufian S, Munoz-Casares FC, Briceno J, et al. Radical surgery—peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol. 2006;94:316–24.
Raspagliesi F, Kusamura S, Torres JCC, et al. Cytoreduction combined with intraperitoneal hyperthermic perfusion chemotherapy in advanced/recurrent ovarian cancer patients: the experience of National Cancer Institute of Milan. Eur J Surg Oncol. 2006;32:671–5.
Helm CW, Toler CR, Martin RS, et al. Cytoreduction and intraperitoneal heated chemotherapy for the treatment of endometrial carcinoma recurrent within the peritoneal cavity. Int J Gynecol Cancer. 2007;17:204–9.
Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113:315–25.
Fagotti A, Paris B, Grimolizzi F, et al. Secondary cytoreduction plus oxaliplatin-based HIPEC in platinum-sensitive recurrent ovarian cancer patients: a pilot study. Gynecol Oncol. 2009;113:335–40.
Author information
Authors and Affiliations
Corresponding author
Additional information
W. P. Ceelen is a Senior Clinical Investigator of the Research Foundation - Flanders (Belgium) (FWO).
Rights and permissions
About this article
Cite this article
Ceelen, W.P., Van Nieuwenhove, Y., Van Belle, S. et al. Cytoreduction and Hyperthermic Intraperitoneal Chemoperfusion in Women with Heavily Pretreated Recurrent Ovarian Cancer. Ann Surg Oncol 19, 2352–2359 (2012). https://doi.org/10.1245/s10434-009-0878-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-009-0878-6