Abstract
Background
Post-gastrectomy weight loss is associated with deterioration in quality of life, and influences the long-term prognosis of gastric cancer patients. We conducted a prospective, randomized controlled, open-label study to examine whether an oral elemental diet (Elental®, Ajinomoto Pharmaceuticals, Tokyo, Japan; hereafter referred to as ED) prevents postoperative weight loss in post-gastrectomy patients.
Methods
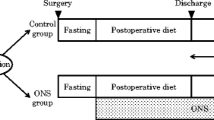
Patients were randomly divided to receive the ED or control diet. The ED group received 300 kcal of ED plus their regular diet for 6–8 weeks after surgery, starting from the day the patient started a soft rice or equivalent diet after surgery, while the control group received the regular diet alone. The primary endpoint was the percentage of body weight loss (%BWL) from the presurgical body weight to that at 6–8 weeks after surgery. Secondary endpoints were dietary adherence, nutrition-related blood parameters, and adverse events.
Results
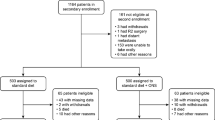
This study included 112 patients in eight hospitals. The mean treatment compliance rate in the ED group was 68.7 ± 30.4 % (median 81.2 %). The %BWL was significantly different between the ED and control groups (4.86 ± 3.72 vs. 6.60 ± 4.90 %, respectively; p = 0.047). In patients who underwent total gastrectomy, the %BWL was significantly different between the two groups (5.03 ± 3.65 vs. 9.13 ± 5.43 %, respectively; p = 0.012). In multivariate analysis, ED treatment, surgery type, and preoperative performance status were independently associated with %BWL. No significant differences were observed in the other clinical variables.
Conclusions
ED supplementation reduced postoperative weight loss in gastric cancer patients undergoing gastrectomy.


Similar content being viewed by others
References
Onodera H, Tokunaga A, Yoshiyuki T, et al. Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology. 2004;51:82–5.
Kong H, Kwon OK, Yu W. Changes of quality of life after gastric cancer surgery. J Gastric Cancer. 2012;12:194–200.
Liu H, Ling W, Shen ZY, Jin X, Cao H. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J Dig Dis. 2012;13:401–6.
Fujitani K, Tsujinaka T, Fujita J, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99:621–9.
Li J, Ji Z, Yuan C, et al. Limited efficacy of early enteral nutrition in patients after total gastrectomy. J Invest Surg. 2011;24:103–8.
Kim HU, Chung JB, Kim CB. The comparison between early enteral nutrition and total parenteral nutrition after total gastrectomy in patients with gastric cancer: the randomized prospective study [in Korean]. Korean J Gastroenterol. 2012;59:407–13.
Rogers C. Postgastrectomy nutrition. Nutr Clin Pract. 2011;26:126–36.
Fukui T, Itoh Y, Orihara M, et al. Elental prevented and reduced oral mucositis during chemotherapy in patients esophageal cancer [in Japanese]. Gan To Kagaku Ryoho 2011;38:2597–601.
Ogata Y, Takeuchi M, Ishibashi N, et al. Efficacy of Elental on prevention for chemotherapy-induced oral mucositis in colorectal cancer patients [in Japanese]. Gan To Kagaku Ryoho. 2012;39:583–7.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20.
Park HS, Jung M, Kim HS, et al. Proper timing of adjuvant chemotherapy affects survival in patients with stage 2 and 3 gastric cancer. Ann Surg Oncol. 2015;22(1):224–31.
Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247:759–65.
Liedman B, Andersson H, Bosaeus I, Hugosson I, Lundell L. Changes in body composition after gastrectomy: results of a controlled, prospective clinical trial. World J Surg. 1997;21:416–20.
Takachi K, Doki Y, Ishikawa O, et al. Postoperative ghrelin levels and delayed recovery from body weight loss after distal or total gastrectomy. J Surg Res. 2006;130:1–7.
Kiyama T, Mizutani T, Okuda T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005;9:313–9.
Abdiev S, Kodera Y, Fujiwara M, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011;14:144–9.
Hirao M, Takiguchi S, Imamura H, et al. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591–7.
Kurokawa Y, Sasako M, Sano T, et al. Functional outcomes after extended surgery for gastric cancer. Br J Surg. 2011;98:239–45.
Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–10.
Bozzetti F, SCRINIO Working Group. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer. 2009;17:279–84.
Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001;29:242–8.
Aoyama T, Yoshikawa T, Shirai J, et al. Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2013;20:2000–6.
Horiuchi A, Nakayama Y, Sakai R, Suzuki M, Kajiyama M, Tanaka N. Elemental diets may reduce the risk of aspiration pneumonia in bedridden gastrostomy-fed patients. Am J Gastroenterol. 2013;108:804–10.
Adachi S, Takiguchi S, Okada K, et al. Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology. 2010;138:1312–20.
Ryan AM, Reynolds JV, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–63.
Yoshikawa T, Hiki N, Taguri M, et al. A phase III trial to evaluate the effect of perioperative nutrition enriched with eicosapentaenoic acid on body weight loss after total gastrectomy for T2-T4a gastric cancer. Jpn J Clin Oncol. 2012;42:459–62.
Papapietro K, Díaz E, Csendes A, et al. Early enteral nutrition in cancer patients subjected to a total gastrectomy. Rev Med Chil. 2002;130:1125–30.
Acknowledgment
The authors would like to thank all KSES collaborators, investigators, and patients for their participation and contribution to this study; Manabu Suzuki, PhD, and Yoshiki Kurose, employees of the Medical Science Department, Ajinomoto Pharmaceuticals Co., Ltd, for providing technical help in data management and writing assistance; Prof. Setsuko Anami, PhD, Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Doshisha Women’s College of Liberal Arts, Kyotanabe, Kyoto, Japan, for her clinical review of adverse events, and proofing and approving the final version of manuscript; Ajinomoto Pharmaceuticals Co., Ltd for providing meeting room facilities; and Nicholas D. Smith, PhD, of Edanz Group Ltd, for providing editorial assistance.
Author Contributions
Conceived and designed the study: Hiroshi Imamura and Ryohei Kawabata. Participated in data acquisition: Hiroshi Imamura, Kazuhiro Nishikawa, Kentaro Kishi, Kentaro Inoue, Jin Matsuyama, Yusuke Akamaru, Yutaka Kimura, Shigeyuki Tamura, Ryohei Kawabata, Junji Kawada, Yoshiyuki Fujiwara, Tomono Kawase, Junichi Fukui, Mari Takagi, and Atsushi Takeno. Statistical analysis and interpretation of data: Hiroshi Imamura. Statistical analysis of data: Toshio Shimokawa. Drafted the article: Hiroshi Imamura. Proofed and approved the final manuscript: Hiroshi Imamura, Kazuhiro Nishikawa, Kentaro Kishi, Yusuke Akamaru, Yutaka Kimura, Shigeyuki Tamura, Ryohei Kawabata, Yoshiyuki Fujiwara, Tomono Kawase, Junichi Fukui, Mari Takagi, Atsushi Takeno, and Toshio Shimokawa. All authors had access to the data and jointly decided to submit the manuscript.
Disclosures
Hiroshi Imamura, Kazuhiro Nishikawa, Kentaro Kishi, Kentaro Inoue, Jin Matsuyama, Yusuke Akamaru, Yutaka Kimura, Shigeyuki Tamura, Ryohei Kawabata, Junji Kawada, Yoshiyuki Fujiwara, Tomono Kawase, Junichi Fukui, Mari Takagi, Atsushi Takeno, and Toshio Shimokawa declare they have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial Registration
UMIN000008056 (University Hospital Medical Information Network Clinical Trials Registry).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Imamura, H., Nishikawa, K., Kishi, K. et al. Effects of an Oral Elemental Nutritional Supplement on Post-gastrectomy Body Weight Loss in Gastric Cancer Patients: A Randomized Controlled Clinical Trial. Ann Surg Oncol 23, 2928–2935 (2016). https://doi.org/10.1245/s10434-016-5221-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5221-4