Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) often is a diagnosis determined by exclusion. Distinguishing ICC from other metastatic adenocarcinomas based on histopathologic or immunohistochemical analysis often is difficult and requires an extensive workup. This study aimed to determine whether albumin, whose expression is restricted to the liver, has potential as a biomarker for ICC using a novel and highly sensitive RNA in situ hybridization (ISH) platform.
Methods
Modified branched DNA probes were developed for albumin RNA ISH. The study evaluated 467 patient samples of primary and metastatic lesions.
Results
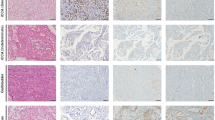
Of the 467 samples evaluated, 83 were ICCs, 42 were hepatocellular carcinomas (HCCs), and 332 were nonhepatic carcinomas including tumors arising from the perihilar region and bile duct, pancreas, stomach, esophagus, colon, breast, ovary, endometrium, kidney, and urinary bladder. Albumin RNA ISH was highly sensitive for cancers of liver origin, staining positive in 82 (99 %) of 83 ICCs and in 42 HCCs (100 %). Perihilar and distal bile duct carcinomas as well as carcinomas arising at other sites tested negative for albumin. Notably, 6 (22 %) of 27 intrahepatic tumors previously diagnosed as carcinomas of undetermined origin tested positive for albumin.
Conclusions
Albumin RNA ISH is a sensitive and highly specific diagnostic tool for distinguishing ICC from metastatic adenocarcinoma to the liver or carcinoma of unknown origin. Albumin RNA ISH could replace the extensive diagnostic workup, leading to timely confirmation of the ICC diagnosis. Additionally, the assay could serve as a guide to distinguish ICC from perihilar adenocarcinoma.




Similar content being viewed by others
References
Everhart JE, Ruhl CE. Burden of Digestive Diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44.
Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–22.
Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–69.
De Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–5.
Malaguarnera G, Paladina I, Giordano M, Malaguarnera M, Bertino G, Berretta M. Serum markers of intrahepatic cholangiocarcinoma. Dis Markers. 2013;34:219–28.
Tickoo SK, Zee SY, Obiekwe S, et al. Combined hepatocellular-cholangiocarcinoma: a histopathologic, immunohistochemical, and in situ hybridization study. Am J Surg Pathol. 2002;26:989.
Krishna M, Lloyd RV, Batts KP. Detection of albumin messenger RNA in hepatic and extrahepatic neoplasms: a marker of hepatocellular differentiation. Am J Surg Pathol. 1997;21:147–52.
Breborowicz J, Tamaoki T. Detection of messenger RNAs of alpha-fetoprotein and albumin in a human hepatoma cell line by in situ hybridization. Cancer Res. 1985;45:1730–6.
Yamaguchi K, Nalesnik MA, Carr BI. In situ hybridization of albumin mRNA in normal liver and liver tumors: identification of hepatocellular origin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:361–5.
D’Errico A, Deleonardi G, Fiorentino M, Scoazec JY, Grigioni WF. Diagnostic implications of albumin messenger RNA detection and cytokeratin pattern in benign hepatic lesions and biliary cystadenocarcinoma. Diagn Mol Pathol. 1998;7:289–94.
Oliveira AMA, Erickson LAL, Burgart LJL, Lloyd RVR. Differentiation of primary and metastatic clear cell tumors in the liver by in situ hybridization for albumin messenger RNA. Am J Surg Pathol 2000;24:177–82.
Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38.
Yan BC, Gong C, Song J, et al. Arginase-1: a new immunohistochemical marker of hepatocytes and hepatocellular neoplasms. Am J Surg Pathol. 2010;34:1147–54.
Voss JS, Holtegaard LM, Kerr SE, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44:1216–22.
Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32:3091–100.
Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552–8.
Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–9.
Muir C. Cancer of unknown primary site. Cancer. 1995;75(1 Suppl):353–6.
Daud AI. Removing the unknown from the carcinoma of unknown primary. J Clin Oncol. 2013;31:174–5.
Borad MJ, Champion MD, Egan JB, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135.
Xiao J-C, Ruck P, Adam A, Wang T-X, Kaiserling E. Small epithelial cells in human liver cirrhosis exhibit features of hepatic stem-like cells: immunohistochemical, electron microscopic and immunoelectron microscopic findings. Histopathology. 2003;42:141–9.
Hainsworth JD, Rubin MS, Spigel DR, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol. 2013;31:217–23.
Monzon FA, Lyons-Weiler M, Buturovic LJ, et al. Multicenter validation of a 1,550-gene expression profile for identification of tumor tissue of origin. J Clin Oncol. 2009;27:2503–8.
Varadhachary G. New strategies for carcinoma of unknown primary: the role of tissue-of-origin molecular profiling. Clin Cancer Res. 2013;19:4027–33.
Acknowledgment
We thank Dr. Gregory Lauwers for critically reviewing this manuscript and Dr. Elena Brachtel and Chin-Lee Wu for contributing tissue microarrays. This work was supported by the Howard Hughes Medical Institute (M.N.R.), the Burroughs Wellcome Trust (D.T.T., M.N.R.), K12CA087723-11A1 (D.T.T.), and Affymetrix, Inc. (D.T.T., M.N.R., V.D.). All the authors have read and approved this version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferrone, C.R., Ting, D.T., Shahid, M. et al. The Ability to Diagnose Intrahepatic Cholangiocarcinoma Definitively Using Novel Branched DNA-Enhanced Albumin RNA In Situ Hybridization Technology. Ann Surg Oncol 23, 290–296 (2016). https://doi.org/10.1245/s10434-014-4247-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4247-8