Abstract
Background
There is conflicting evidence concerning platelet status and hepatocellular carcinoma (HCC) prognosis. We evaluated the prognostic value of platelet-based indices, including platelet count, platelet/lymphocyte ratio (PLR), and aspartate aminotransferase to platelet ratio index (APRI) in HCC after hepatic resection.
Methods
We retrospectively reviewed 332 patients with HCC treated with hepatectomy between 2006 and 2009. Preoperative platelet count, as well as demographic, clinical, and pathologic data, were analyzed.
Results
Both disease-free survival (DFS) and overall survival (OS) were significantly improved for patients with low platelet count, PLR, and APRI compared to patients with elevated values. On multivariate analysis, APRI, tumor size ≥5 cm, noncapsulation, and multiple tumors were all associated with both poor DFS and OS. The 1-, 3-, and 5-year DFS rates were 52, 36, and 32 % for patients with APRI <0.62 and were 35, 22, and 19 % for patients with APRI ≥0.62. Correspondingly, the 1-, 3-, and 5-year OS rates were 77, 51, and 42, and 63, 35, and 29 % for both groups. Both DFS and OS of patients with APRI <0.62 were significantly better compared to patients with an elevated APRI (P = 0.009 and 0.002, respectively). Patients with elevated APRI tended to have cirrhosis, hepatitis B virus (HBV) infection, surgical margin <1 cm, and noncapsulated tumors.
Conclusions
Elevated platelets based inflammatory indices, especially APRI, was associated with adverse characteristic features and poor prognosis in HCC, especially for patients with HBV infection or cirrhosis. Antiplatelet treatment may represent a potential therapy for HBV-induced HCC recurrence.



Similar content being viewed by others
References
Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–63.
Ercolani G, Grazi GL, Ravaioli M, Del GM, Gardini A, Cescon M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–43.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55.
Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139:755–64.
Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–62.
Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet–lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72.
Sitia G, Aiolfi R, Di LP, Mainetti M, Fiocchi A, Mingozzi F, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci USA. 2012;109:E2165–72.
Kurt M, Onal IK, Sayilir AY, Beyazit Y, Oztas E, Kekilli M, et al. The role of mean platelet volume in the diagnosis of hepatocellular carcinoma in patients with chronic liver disease. Hepatogastroenterology. 2012;59:1580–2.
Maithel SK, Kneuertz PJ, Kooby DA, Scoggins CR, Weber SM, Martin RC II, et al. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi-institutional analysis. J Am Coll Surg. 2011;212:638–48.
Ichikawa T, Uenishi T, Takemura S, Oba K, Ogawa M, Kodai S, et al. A simple, noninvasively determined index predicting hepatic failure following liver resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:42–8.
Amano H, Tashiro H, Oshita A, Kobayashi T, Tanimoto Y, Kuroda S, et al. Significance of platelet count in the outcomes of hepatectomized patients with hepatocellular carcinoma exceeding the Milan criteria. J Gastrointest Surg. 2011;15:1173–81.
Hwang SJ, Luo JC, Li CP, Chu CW, Wu JC, Lai CR, et al. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:2472–7.
Gao J, Zhang HY, Xia YF. Increased platelet count is an indicator of metastasis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013;34:39–45.
Hwang SG, Kim KM, Cheong JH, Kim HI, An JY, Hyung WJ, et al. Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol. 2012;38:562–7.
Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM. 2006;8:19–20.
Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–33.
He MM, Zhang DS, Wang F, Wang ZQ, Luo HY, Jin Y, et al. The role of non-curative surgery in incurable, asymptomatic advanced gastric cancer. PLoS One. 2013;8:e83921. doi:10.1371/journal.pone.0083921.
Lin MS, Huang JX, Zhu J, Shen HZ. Elevation of platelet count in patients with colorectal cancer predicts tendency to metastases and poor prognosis. Hepatogastroenterology. 2012;59:1687–90.
Gorelick C, Andikyan V, Mack M, Lee YC, Abulafia O. Prognostic significance of preoperative thrombocytosis in patients with endometrial carcinoma in an inner-city population. Int J Gynecol Cancer. 2009;19:1384–9.
Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13:499–503.
Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23:265–73.
Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–36.
Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691–9.
Kao WY, Chiou YY, Hung HH, Chou YH, Su CW, Wu JC, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase–platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23:528–36.
Sabrkhany S, Griffioen AW, Oude EMG. The role of blood platelets in tumor angiogenesis. Biochim Biophys Acta. 2011;1815:189–96.
Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30:2362–7.
Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis. Lancet. 1998;352:1775–7.
Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95:9325–30.
Maini MK, Schurich A. Platelets harness the immune response to drive liver cancer. Proc Natl Acad Sci USA. 2012;109:12840–1.
Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–300.
Choe KS, Correa D, Jani AB, Liauw SL. The use of anticoagulants improves biochemical control of localized prostate cancer treated with radiotherapy. Cancer. 2010;116:1820–6.
Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149–61.
Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–5.
van Doormaal FF, Di NM, Otten HM, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol. 2011;29:2071–6.
Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–9.
Acknowledgment
We thank Professor Stephen Tomlinson from the Department of Microbiology and Immunology, Medical University of South Carolina, for his review and comments.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shun-Li Shen and Shun-Jun Fu have contributed equally to this article, and both should be considered first author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
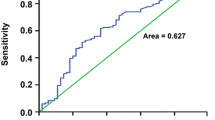
Supplement Fig. 1
Receiver operating characteristic curves of preoperative PLR (a) and APRI (b) for predicting tumor recurrence in patients with hepatocellular carcinoma (HCC) after hepatic resection (TIF 113 kb)
Supplement Fig. 2
Kaplan-Meier survival curves of HCC patients with after hepatectomy. The patients were divided into platelet <300/mm3 group and platelet ≥300/mm3 group. a Disease-free survival of patients with platelet ≥300/mm3 was shorter than those with platelet <300/mm3 (P = .009, log-rank). b Overall survival of patients with platelet ≥300/mm3 was also shorter than those with platelet <300/mm3 (P = .02, log-rank). (TIF 148 kb)
Supplement Fig. 3
Kaplan--Meier survival curves of HCC patients after hepatectomy. The patients were divided into PLR <115 group and PLR ≥115 group by the optimal cut-off value of PLR. a Disease-free survival of patients with PLR ≥115 was shorter than those with PLR <115 (P = .026, log-rank). b Overall survival of patients with PLR ≥115 was also shorter than those with PLR <115 (P = .007, log-rank). (TIF 151 kb)
Rights and permissions
About this article
Cite this article
Shen, SL., Fu, SJ., Chen, B. et al. Preoperative Aspartate Aminotransferase to Platelet Ratio is an Independent Prognostic Factor for Hepatitis B-Induced Hepatocellular Carcinoma After Hepatic Resection. Ann Surg Oncol 21, 3802–3809 (2014). https://doi.org/10.1245/s10434-014-3771-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3771-x