Abstract
Background
Currently, complete surgical resection is the only curative option for medullary thyroid cancer (MTC). Previous work has shown the Notch pathway is a potent tumor suppressor in MTC and that resveratrol activates the Notch pathway in carcinoid cancer, a related neuroedocrine malignancy. In this study, we hypothesized that the effects observed on carcinoid cells could be extended to MTC.
Methods
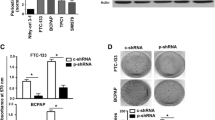
MTC cells treated with varying doses of resveratrol were assayed for viability by the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay. Western blot analysis for achaete-scute complex-like 1 (ASCL1), chromogranin A (CgA), full-length and cleaved caspase 3, and poly-ADP ribose polymerase (PARP) was performed. Quantitative real-time polymerase chain reaction (qPCR) was used to measure relative mRNA expression.
Results
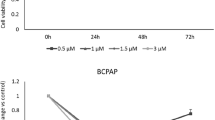
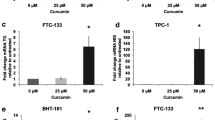
Treatment with resveratrol resulted in growth suppression and an increase in the cleavage of caspase-3 and PARP. A dose-dependent inhibition of ASCL1, a neuroedocrine transcription factor, was observed at the protein and mRNA levels. Protein levels of CgA, a marker of hormone secretion, were also reduced after treatment with resveratrol. A dose-dependent induction of Notch2 mRNA was observed by qPCR.
Conclusions
Resveratrol suppresses in vitro growth, likely through apoptosis, as demonstrated by cleavage of caspase-3 and PARP. Furthermore, resveratrol decreased neuroedocrine markers ASCL1 and chromogranin A. Induction of Notch2 mRNA suggests that this pathway may be central in the anti-MTC effects observed.




Similar content being viewed by others
References
Sippel RS, Carpenter JE, Kunnimalaiyaan M, et al. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery. 2003;134:866–71.
Giovanella L, Crippa S, Cariani L. Serum calcitonin-negative medullary thyroid carcinoma: role of CgA and CEA as complementary markers. Int J Biol Markers. 2008;23:129–31.
Seregni E, Ferrari L, Bajetta E, et al. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S69–72.
Tomassetti P, Migliori M, Simoni P, et al. Diagnostic value of plasma chromogranin A in neuroendocrine tumours. Eur J Gastroenterol Hepatol. 2001;13:55–8.
Chen H, Hardacre J, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88–92.
Chen H, Kunnimalaiyaan M, Van Gompel JJ. Medullary thyroid cancer: the functions of raf-1 and human achaete-scute homologue-1. Thyroid. 2005;15:511–21.
Giuffrida D, Gharib H. Current diagnosis and management of medullary thyroid carcinoma. Ann Oncol. 1998;9:695–701.
Quayle F, Moley J. Medullary thyroid carcinoma: including MEN 2A and MEN 2B syndromes. J Surg Oncol. 2005;89:122–9.
Pelizzo M, Boschin I, Bernante P, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol. 2007;33:493–7.
Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist. 2007;12:942–51.
Janson E, Holmberg L, Stridsberg M, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol. 1997;8:685–90.
Ball DW. Achaete-scute homolog-1 and Notch in lung neuroendocrine development and cancer. Cancer Lett. 2004;204:159–69.
Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, et al. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–30.
Greenblatt DY, Cayo MA, Adler JT, et al. Valproic acid activates Notch1 signaling and induces apoptosis in medullary thyroid cancer cells. Ann Surg. 2008;247:1036–40.
Adler J, Hottinger D, Kunnimalaiyaan M, et al. Inhibition of growth in medullary thyroid cancer cells with histone deacetylase inhibitors and lithium chloride. J Surg Res. 2010;159:640–4.
Ning L, Greenblatt DY, Kunnimalaiyaan M, et al. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13:98–104.
Ning L, Jaskula-Sztul R, Kunnimalaiyaan M, et al. Suberoyl bishydroxamic acid activates notch1 signaling and suppresses tumor progression in an animal model of medullary thyroid carcinoma. Ann Surg Oncol. 2008;15:2600–5.
Pinchot SN, Jaskula-Sztul R, Ning L, et al. Identification and validation of Notch pathway activating compounds through a novel high-throughput screening method. Cancer. (in press).
Baur J, Sinclair D. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506.
Juan M, Vinardell M, Planas J. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J Nutr. 2002;132:257–60.
Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila). 2009;2:409–18.
Schimmer A. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64:7183–90.
Jiang C, Wang Z, Ganther H, et al. Caspases as key executors of methyl selenium–induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61:3062–70.
Garnier P, Ying W, Swanson R. Ischemic preconditioning by caspase cleavage of poly(ADP-ribose) polymerase-1. J Neurosci. 2003;23:7967–73.
Van Gompel JJ, Sippel RS, Warner TF, et al. Gastrointestinal carcinoid tumors: factors that predict outcome. World J Surg. 2004;28:387–92.
Jiang S, Kameya T, Asamura H, et al. hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol. 2004;17:222–9.
Kunnimalaiyaan M, Ndiaye M, Chen H. Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery. 2006;140:1009–14.
Bhattacharyya N. A population-based analysis of survival factors in differentiated and medullary thyroid carcinoma. Otolaryngol Head Neck Surg. 2003;128:115–23.
Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–5.
Chen H, Biel MA, Borges MW, et al. Tissue-specific expression of human achaete-scute homologue-1 in neuroendocrine tumors: transcriptional regulation by dual inhibitory regions. Cell Growth Differ. 1997;8:677–86.
Acknowledgment
Supported in part by Department of Surgery T35 Short Term Training Grant (DK 062709-0401 to M.T.), Howard Hughes Medical Institute (to M.R.C.), National Institutes of Health (Grants RO1 CA121115 and RO1 CA109053 to H.C.), and American College of Surgeons George H. A. Clowes Jr. Memorial Research Career Development Award (to H.C.), and Carcinoid Cancer Foundation Research Award (to H.C.).
Conflicts of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Truong, M., Cook, M.R., Pinchot, S.N. et al. Resveratrol Induces Notch2-Mediated Apoptosis and Suppression of Neuroendocrine Markers in Medullary Thyroid Cancer. Ann Surg Oncol 18, 1506–1511 (2011). https://doi.org/10.1245/s10434-010-1488-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1488-z