Abstract
Background
Although only a small proportion of thin melanomas result in lymph node metastasis, the abundance of these lesions results in a relatively large absolute number of patients with a diagnosis of nodal metastases, determined by either sentinel lymph node (SLN) biopsy or clinical nodal recurrence (CNR).
Methods
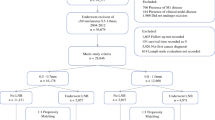
Independent cohorts with thin melanoma and either SLN metastasis or CNR were identified at two melanoma referral centers. At both centers, SLN metastasis patients were included. At center 1, the CNR cohort included patients with initial negative clinical nodal evaluation followed by CNR. At center 2, the CNR cohort was restricted to those presenting in the era before the use of SLN biopsy. Uni- and multivariable analyses of melanoma-specific survival (MSS) were performed.
Results
At center 1, 427 CNR patients were compared with 91 SLN+ patients. The 5- and 10-year survival rates in the SLN group were respectively 88 and 84 % compared with 72 and 49 % in the CNR group (p < 0.0001). The multivariate analysis showed age older than 50 years (hazard ratio [HR] 1.5; 95 % confidence interval [CI] 1.2–1.9), present ulceration (HR 1.9; 95 % CI 1.2–2.9), unknown ulceration (HR 1.6; 95 % CI 1.3–2.1), truncal site (HR 1.6; 95 % CI 1.2–2.2), and CNR (HR 3.3; 95 % CI 1.8–6.0) to be associated significantly with decreased MSS (p < 0.01 for each). The center 2 cohort demonstrated remarkably similar findings, with a 5-year MSS of 88 % in the SLN (n = 29) group and 76 % in the CNR group (n = 39, p = 0.09).
Conclusion
Patients with nodal metastases from thin melanomas have a substantial risk of melanoma death. This risk is lower among patients whose disease is discovered by SLN biopsy rather than CNR.



Similar content being viewed by others
References
Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO Melanoma Programme. Lancet. 1998;351:793–6.
Balch CM, Soong S, Ross MI, Urist MM, Karakousis CP, Temple WJ, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87–97.
Gimotty PA, Guerry D, Ming ME, Elenitsas R, Xu X, Czerniecki B, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22:3668–76.
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206.
Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute, Bethesda, MD, 2011. http://seer.cancer.gov/csr/1975_2009_pops09/.
Wong SL, Balch CM, Hurley P, Agarwala SS, Akhurst TJ, Cochran A, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30:2912–8.
Plitas G, Ariyan CE. Controversies in the management of regional nodes in melanoma. J Natl Compr Cancer Netw. 2012;10:414–21.
Ra JH, McMasters KM, Spitz FR. Should all melanoma patients undergo sentinel lymph node biopsy? Curr Opin Oncol. 2006;18:185–8.
Karakousis GC, Gimotty PA, Botbyl JD, Kesmodel SB, Elder DE, Elenitsas R, et al. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol. 2006;13:533–41.
Faries MB, Wanek LA, Elashoff D, Wright BE, Morton DL. Predictors of occult nodal metastasis in patients with thin melanoma. Arch Surg. 2010;145:137–42.
Warycha MA, Zakrzewski J, Ni Q, Shapiro RL, Berman RS, Pavlick AC, Polsky D, et al. Meta-analysis of sentinel lymph node positivity in thin melanoma (≤1 mm). Cancer. 2009;115:869–79.
Karakousis GC, Gimotty PA, Czerniecki BJ, Elder DE, Elenitsas R, Ming ME, et al. Regional nodal metastatic disease is the strongest predictor of survival in patients with thin vertical growth phase melanomas: a case for SLN staging biopsy in these patients. Ann Surg Oncol. 2007;14:1596–603.
Wright BE, Scheri RP, Ye X, Faries MB, Turner RR, Essner R Morton DL. Importance of sentinel lymph node biopsy in patients with thin melanoma. Arch Surg. 2008;143:892–9; discussion 9–900.
Ranieri JM, Wagner JD, Wenck S, Johnson CS Coleman JJ III. The prognostic importance of sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol. 2006;13:927–32.
Bartlett EK, Gimotty PA, Sinnamon AJ, Wachtel H, Roses RE, Schuchter L, et al. Clark level risk stratifies patients with mitogenic thin melanomas for sentinel lymph node biopsy. Ann Surg Oncol. 2014;21:643–9.
Han D, Zager JS, Shyr Y, Chen H, Berry LD, Iyengar S, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31:4387–93.
Murali R, Haydu LE, Quinn MJ, Saw RP, Shannon K, Spillane AJ, et al. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg. 2012;255:128–33.
Wong SL, Brady MS, Busam KJ, Coit DG. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol. 2006;13:302–9.
Gajdos C, Griffith KA, Wong SL, Johnson TM, Chang AE, Cimmino VM, et al. Is there a benefit to sentinel lymph node biopsy in patients with T4 melanoma? Cancer. 2009;115:5752–60.
Gershenwald JE, Mansfield PF, Lee JE, Ross MI. Role for lymphatic mapping and sentinel lymph node biopsy in patients with thick (≥4 mm) primary melanoma. Ann Surg Oncol. 2000;7:160–5.
Thompson JF Shaw HM. The prognosis of patients with thick primary melanomas: is regional lymph node status relevant, and does removing positive regional nodes influence outcome? Ann Surg Oncol. 2002;9:719–22.
Carlson GW, Murray DR, Hestley A, Staley CA, Lyles RH Cohen C. Sentinel lymph node mapping for thick (≥4 mm) melanoma: should we be doing it? Ann Surg Oncol. 2003;10:408–15.
Morton DL, Thompson JF, Essner R, Elashoff R, Stern SL, Nieweg OE, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230:453–63; discussion 63–5.
Faries MB, Steen S, Ye X, Sim M Morton DL. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27–34.
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014; 370:599–609.
Acknowledgment
This study was supported by Grants P50-CA093372, P30-CA016520, P01-CA29605, and R01 CA189163 from the National Cancer Institute, the Dr. Miriam & Sheldon G. Adelson Medical Research Foundation (Boston, MA, USA), the Borstein Family Foundation, (Los Angeles, CA, USA), and the John Wayne Cancer Institute Auxiliary (Santa Monica, CA, USA). The content of this report is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Conflicts of interest
The authors have no conflicts of interest to disclose regarding the contents of this study or its publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karakousis, G., Gimotty, P.A., Bartlett, E.K. et al. Thin Melanoma with Nodal Involvement: Analysis of Demographic, Pathologic, and Treatment Factors with Regard to Prognosis. Ann Surg Oncol 24, 952–959 (2017). https://doi.org/10.1245/s10434-016-5646-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5646-9