Abstract
Purpose
To determine U.S. treatment patterns for pathologic staging practices in patients with thick head and neck melanomas (HNM).
Methods
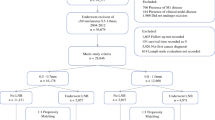
Patients with thick HNM without clinical evidence of in-transit, regional, or distant metastatic spread at presentation were identified from the Surveillance Epidemiology and End Results database. Treatment trends for patients were summarized, and univariate and multivariate analyses were performed to identify associations between varying practice patterns.
Results
A total of 1,230 patients with HNM meeting the inclusion criteria were identified. Surgical staging procedures were utilized in 53.5 %, including both sentinel lymph node biopsy (37 %) and elective neck dissection (16 %). Patients undergoing a surgical staging procedure were younger (64 vs. 77 years, p < 0.001) with smaller tumors (6.3 vs. 6.6 mm, p = 0.008). The rate of occult nodal disease was 22 % in patients undergoing a surgical staging procedure. The presence of a positive regional node in this subgroup of patients was associated with a significant reduction in disease-specific (44 vs. 59 months, p < 0.001) and overall survival (40 vs. 53 months, p < 0.001) on univariate analysis. On multivariate analysis, the presence of a positive node was the most significant factor for reduced overall survival (hazard ratio 2.36, 95 % confidence interval 1.71–3.23) and disease-specific survival (hazard ratio 2.84, 95 % confidence interval 1.99–4.06).
Conclusions
Pathologic staging procedures provide independent prognostic information for patients with thick HNM. Despite this, current practice patterns demonstrate underutilization, particularly in elderly patients. Further work is needed to address the barriers to pathologic staging implementation in patients with thick HNM.

Similar content being viewed by others
References
Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17.
Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250–75.
Wong SL, Balch CM, Hurley P, et al. Sentinel lymph node biopsy for melanoma: American Society of Clinical Oncology and Society of Surgical Oncology joint clinical practice guideline. J Clin Oncol. 2012;30:2912–8.
Perrott RE, Glass LF, Reintgen DS, Fenske NA. Reassessing the role of lymphatic mapping and sentinel lymphadenectomy in the management of cutaneous malignant melanoma. J Am Acad Dermatol. 2003;49:567–88.
Essner R, Chung MH, Bleicher R, Hsueh E, Wanek L, Morton DL. Prognostic implications of thick (> or = 4-mm) melanoma in the era of intraoperative lymphatic mapping and sentinel lymphadenectomy. Ann Surg Oncol. 2002;9:754–61.
Gajdos C, Griffith KA, Wong SL, et al. Is there a benefit to sentinel lymph node biopsy in patients with T4 melanoma? Cancer. 2009;115:5752–60.
Carlson GW, Murray DR, Hestley A, Staley CA, Lyles RH, Cohen C. Sentinel lymph node mapping for thick (> or = 4-mm) melanoma: should we be doing it? Ann Surg Oncol. 2003;10:408–15.
Cecchi R, Buralli L, Innocenti S, Seghieri G, De Gaudio C. Sentinel lymph node biopsy in patients with thick (=4 mm) melanoma: a single-centre experience. J Eur Acad Dermatol Venereol. 2007;21:758–61.
Cherpelis BS, Haddad F, Messina J, et al. Sentinel lymph node micrometastasis and other histologic factors that predict outcome in patients with thicker melanomas. J Am Acad Dermatol. 2001;44:762–6.
Covarelli P, Vedovati MC, Becattini C, et al. The sentinel node biopsy in patients with thick melanoma: outcome analysis from a single-institution database. In Vivo. 2011;25:439–43.
Fairbairn NG, Orfaniotis G, Butterworth M. Sentinel lymph node biopsy in thick malignant melanoma: a 10-year single unit experience. J Plast Reconstr Aesthet Surg. 2012;65:1396–402.
Ferrone CR, Panageas KS, Busam K, Brady MS, Coit DG. Multivariate prognostic model for patients with thick cutaneous melanoma: importance of sentinel lymph node status. Ann Surg Oncol. 2002;9:637–45.
Fujisawa Y, Otsuka F. The benefit of a sentinel lymph node biopsy and adjuvant therapy in thick (>4 mm) melanoma: multicenter, retrospective study of 291 Japanese patients. Melanoma Res. 2012;22:362–7.
Gershenwald JE, Mansfield PF, Lee JE, Ross MI. Role for lymphatic mapping and sentinel lymph node biopsy in patients with thick (>or = 4 mm) primary melanoma. Ann Surg Oncol. 2000;7:160–5.
Goppner D, Ulrich J, Pokrywka A, Peters B, Gollnick H, Leverkus M. Sentinel lymph node biopsy status is a key parameter to stratify the prognostic heterogeneity of malignant melanoma in high-risk tumors >4.0 mm. Dermatology. 2011;222:59–66.
Gutzmer R, Satzger I, Thoms KM, et al. Sentinel lymph node status is the most important prognostic factor for thick (> or = 4 mm) melanomas. J Dtsch Dermatol Ges. 2008;6:. 198–203.
Jacobs IA, Chang CK, Salti GI. Role of sentinel lymph node biopsy in patients with thick (> 4 mm) primary melanoma. Am Surg. 2004;70:59–62.
Kelly J, Redmond HP. The role of sentinel lymph node biopsy in patients with thick melanoma. A single centre experience. Surgeon. 2012;10:65–70.
Meguerditchian AN, Asubonteng K, Young C, Lema B, Wilding G, Kane JM 3rd. Thick primary melanoma has a heterogeneous tumor biology: an institutional series. World J Surg Oncol. 2011;9:40.
Rondelli F, Vedovati MC, Becattini C, et al. Prognostic role of sentinel node biopsy in patients with thick melanoma: a meta-analysis. J Eur Acad Dermatol Venereol. 2012;26:560–5.
Rughani MG, Swan MC, Adams TS, et al. Sentinel node status predicts survival in thick melanomas: the Oxford perspective. Eur J Surg Oncol. 2012;38:936–42.
Scoggins CR, Bowen AL, Martin RC 2nd, et al. Prognostic information from sentinel lymph node biopsy in patients with thick melanoma. Arch Surg. 2010;145:622–7.
Thompson JF, Shaw HM. The prognosis of patients with thick primary melanomas: is regional lymph node status relevant, and does removing positive regional nodes influence outcome? Ann Surg Oncol. 2002;9:719–22.
Miller MW, Vetto JT, Monroe MM, Weerasinghe R, Andersen PE, Gross ND. False-negative sentinel lymph node biopsy in head and neck melanoma. Otolaryngol Head Neck Surg. 2011;145:606–11.
Cormier JN, Xing Y, Ding M, et al. Population-based assessment of surgical treatment trends for patients with melanoma in the era of sentinel lymph node biopsy. J Clin Oncol. 2005;23:6054–62.
Reyes-Ortiz CA, Goodwin JS, Zhang DD, Freeman JL. Socioeconomic status and chemotherapy use for melanoma in older people. Can J Aging. 2011;1:1–11.
Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–69.
Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30:972–9.
Dragun AE, Huang B, Tucker TC, Spanos WJ. Disparities in the application of adjuvant radiotherapy after breast-conserving surgery for early stage breast cancer: impact on overall survival. Cancer. 2011;117:2590–8.
Ortholan C, Benezery K, Dassonville O, et al. A specific approach for elderly patients with head and neck cancer. Anticancer Drugs. 2011;22:647–55.
Reizenstein JA, Bergstrom SN, Holmberg L, et al. Impact of age at diagnosis on prognosis and treatment in laryngeal cancer. Head Neck. 2010;32:1062–8.
Foster JA, Salinas GD, Mansell D, Williamson JC, Casebeer LL. How does older age influence oncologists’ cancer management? Oncologist. 2010;15:584–92.
Quipourt V, Jooste V, Cottet V, Faivre J, Bouvier AM. Comorbidities alone do not explain the undertreatment of colorectal cancer in older adults: a French population-based study. J Am Geriatr Soc. 2011;59:694–8.
Monroe MM, Gross ND. Evidence-based practice: management of the clinical node-negative neck in early-stage oral cavity squamous cell carcinoma. Otolaryngol Clin North Am. 2012;45:1181–93.
National Commission on Cancer. Scope of regional lymph node surgery: a review of data validity, revised coding directives, and agency transition plans. http://www.facs.org/cancer/ncdb/scope-regional-lymph-node-surgery.pdf. Accessed 26 Feb 2013.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monroe, M.M., Myers, J.N. & Kupferman, M.E. Undertreatment of Thick Head and Neck Melanomas: An Age-based Analysis. Ann Surg Oncol 20, 4362–4369 (2013). https://doi.org/10.1245/s10434-013-3160-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-013-3160-x