Abstract
Purpose
We conducted a phase II trial to assess the survival duration and quality of life of patients who received adjuvant interferon-based chemoradiation for pancreatic adenocarcinoma after pancreaticoduodenectomy.
Methods
Patients with a performance status of 0 or 1 were enrolled to receive interferon-alfa-2b (3 million units MWF), cisplatin (30 mg/m2, 6 doses) and 5-fluorouracil (5-FU; 175 mg/m2/day), concurrent with external-beam radiation (50.4 Gy) and followed by 2 courses of systemic 5-FU. The protocol was modified to include an optional 9 day break in the middle of chemoradiation. Quality of life was assessed by use of validated instruments.
Results
Twenty-eight patients were eligible for analysis. The operation of 15 (54%) patients was performed at other institutions. All patients had T3 tumors, 22 (79%) had positive lymph nodes and 4 (14%) had positive (R1) margins. 24 (86%) patients completed therapy. In all, 25 (89%) patients experienced grade 3 toxicity and 3 (11%) patients were hospitalized. The most common grade 3 events were leukopenia (15, 54%) and neutropenia (12, 43%). No grade 4 toxicity occurred. Overall quality of life decreased during chemoradiation but returned to baseline thereafter and was stable throughout surveillance. 19 patients have died; the median follow-up of the 9 survivors is 62 months. The median OS duration of treated patients was 42.3 (95% confidence interval 30.5–54.2) months.
Conclusions
Adjuvant interferon-based chemoradiation can be delivered safely and tolerably—though with substantial reversible toxicity—to patients of good performance status at an experienced cancer center. Therapy may be associated with an improvement in overall survival.
Similar content being viewed by others
Adjuvant therapy improves the median overall survival (OS) duration of patients with potentially resectable pancreatic ductal adenocarcinoma (PDAC) compared to surgery alone.1–3 Despite the completion of numerous trials evaluating postoperative therapy regimens for patients with PDAC, however, no meaningful further improvements in survival have been observed since the first multi-institutional trial of adjuvant chemoradiation was reported in 1985.1 At present, the median survival of patients treated postoperatively with standard 5-fluorouracil (5-FU)- or gemcitabine-based chemotherapy with or without chemoradiation is 20–23 months.3,4
Over the past decade, attention has focused on a promising chemoradiation regimen that includes a biologic response modifier. Interferon-based chemoradiation was first evaluated as adjuvant therapy for patients with resectable PDAC at Virginia Mason Hospital and Medical Center.5,6 Interferon alfa-2b has synergistic activity with 5-FU and cisplatin and all three agents have radiosensitizing properties.7–11 A continuous infusion of 5-FU, bolus cisplatin, and subcutaneous injections of interferon alfa-2b were therefore administered concurrently with 45.0–54 Gy of external-beam radiation after surgery. Chemoradiation was followed by systemic 5-FU. The first group of patients treated with this regimen had a median OS of at least 32 months and a 5-year OS rate of 55%.6 A multi-institutional, single-arm phase II study of this regimen was subsequently conducted by the American College of Surgeons Oncology Group (ACOSOG Z5031); the median OS in that trial was 27.1 months.12 More recently, an even longer median OS—32.1 months—was observed among patients treated with adjuvant interferon-based chemoradiation in the European phase III Combined chemoradioimmunotherapy for Pancreatic Adenocarcinoma (CapRI) trial.13 Although this remains the longest median OS duration ever reported in a multi-institutional trial of adjuvant therapy for patients with PDAC, it was not greatly different from the 28.5-month median OS of patients treated with adjuvant systemic 5-FU observed in that study.
Despite these encouraging data, concerns exist about the toxicities associated with interferon-based chemoradiation. Indeed, rates of grade 3 and 4 toxicity reported in studies to date have been 68–95%.6,12–14 In the ACOSOG study, the high incidence of adverse events led to its premature closure (89 of 93 patients).12 All adverse events have been reversible, however, and no treatment-related deaths have ever been reported. Moreover, the overall quality of life (QOL) of patients treated with interferon-based chemoradiation in the CapRI trial was similar to that of patients treated with systemic 5-FU.13
To further characterize the survival rates, toxicity rates and QOL associated with treatment with adjuvant interferon-based chemoradiation we conducted a phase II trial. We incorporated a rigorous QOL study to evaluate the impact of this regimen upon patients’ perception of their own general well-being, treatment- and pancreatic cancer-specific symptoms, and mental health.
Patients and Methods
Patients and Eligibility
The University of Texas M. D. Anderson Cancer Center Institutional Review Board approved this study, and all participants gave written informed consent. The study was open from September, 2002 through October, 2008. Patients were required to be at least 18 years of age and to have biopsy-proven, completely resected (R0 or R1) adenocarcinomas of the pancreatic head. Surgical resection need not have been performed at M. D. Anderson Cancer Center. A postoperative computed tomography (CT) study demonstrating an absence of residual disease was required. Patients were also required to have an Eastern Cooperative Group performance status of 0 or 1, a creatinine level ≤1.5 mg/dl or measured creatinine clearance ≥60 ml/min, a hemoglobin level >9.0 g/dl, and white blood cell, absolute neutrophil, and platelet counts >3000, 1500, and 75,000 cells/μl, respectively. Chemoradiation was required to begin within 12 weeks of surgery.
Treatment Protocol
The initial treatment scheme is depicted in Fig. 1a. 5-FU was administered by continuous intravenous infusion of 175 mg/m2/day concurrently with external-beam radiation (50.4 Gy in 28 1.8-Gy fractions) 5 days/week for 5.5 weeks. Cisplatin was given as a 30 mg/m2 intravenous bolus once a week for 6 weeks. Interferon alfa-2b (3 million units) was given subcutaneously on Monday, Wednesday, and Friday each week for 6 weeks (17 doses).
The radiation treatment volume included the pancreatic tumor bed, the porta hepatis, and the origins of the celiac axis and superior mesenteric artery. A four-field technique with customized blocking was employed. The field was reduced off of the porta hepatis after 45 Gy for the final 5.4 Gy.
Because of the toxicities observed in the first 11 patients treated, the protocol was modified before treatment of the 12th patient to include an optional 9-day treatment break from all therapy on day 20, as depicted in Supplementary Fig. 1b.
Two cycles of systemic 5-FU were administered beginning 4–6 weeks after completion of chemoradiation. Patients received treatments (200 mg/m2/day) continuously for 6 weeks with 2 weeks of rest between cycles.
Adverse Events
Adverse events were graded with the Common Terminology Criteria for Adverse Events, version 3.0.15 Decisions regarding chemoradiation treatment and chemotherapy dose adjustments were made on the basis of hematologic assays and clinical assessments performed weekly during chemoradiation and every 2–3 weeks thereafter. Patients who experienced progressive gastrointestinal toxicities were administered outpatient intravenous hydration as needed. Grade 3 or 4 gastrointestinal and hematologic toxicities required interruption of all chemoradiation treatments until toxicity resolved or returned to a grade 1 level (7 days minimum). When chemoradiation was resumed, 5-FU and cisplatin doses were reduced by 25%.
Follow-up
After completion of all therapy, patients were evaluated every 2–4 months for the first 2 years, every 3–5 months for the third year, and every 5–7 months for the fourth and fifth years. Thereafter, patients were evaluated on an annual basis. Each evaluation included a complete physical examination, chest radiograph, abdominal CT, and serum laboratory studies including CA 19-9. Biopsy confirmation of recurrence was not required.
QOL Instruments
Three QOL instruments were administered to each patient upon enrollment (baseline), at the midpoint of and immediately after completion of chemoradiation (weeks 3 and 6), before each course of systemic 5-FU, and at each surveillance evaluation.
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 is a 30-item measure of QOL.16 The instrument evaluates functional status, general symptoms and global health. It has been used with a variety of cancer patients and has good internal consistency and good test-retest reliability.17
The EORTC Quality of Life Questionnaire-Pancreas 26 was designed for use with the C30 to assess pancreatic cancer-specific symptoms, such as pain, gastrointestinal distress, and body image.18
The Center for Epidemiologic Studies-Depression is a 20-item measure of depression that has demonstrated high internal consistency and convergent validity with other measures of depression.19
Off-protocol Therapy
To place the data from this trial into context, clinical data for patients who underwent pancreaticoduodenectomy and were not treated on this protocol were obtained from our prospectively maintained pancreatic tumor database.20
Statistical Analysis
OS and disease-free survival (DFS) were calculated from the date of surgery by the Kaplan–Meier method. OS was defined as the time from surgery to death or last follow-up. DFS was defined as the time from surgery to first disease recurrence or death. The log-rank test was used to assess differences in survival curves. Univariate Cox proportional hazards models were fit to evaluate the effect of potential prognostic factors upon DFS and OS. Each QOL instrument was analyzed by evaluating the mean, standard deviation, median, and range of self-reported scores for each item; the Wilcoxon signed rank test was used to assess differences in the scores between baseline and points of interest. SAS 9.2 (SAS Institute, Cary, NC) was used for statistical analyses. P ≤ 0.05 was considered significant.
Results
Patients
Twenty-nine patients were enrolled. One patient withdrew from the study before initiation of treatment. Of 28 patients who received therapy, 13 (46%) had undergone pancreaticoduodenectomy at M. D. Anderson and 15 (54%) had received surgery elsewhere. All patients were reported to have had potentially resectable cancers before surgery. The clinicopathologic characteristics of all 28 patients are reported in Table 1.
Chemoradiation, Chemotherapy, and Toxicity
The full dose of 50.4 Gy of radiation was administered to 24 patients (86%); 3 patients withdrew from the study before completion of chemoradiation; and 1 patient received only 43.2 Gy but remained in the study. Seventeen patients (61%) required a reduction of chemotherapy doses during chemoradiation. Of the 25 patients who began systemic chemotherapy, 1 did not complete it, secondary to the interval development of metastatic disease.
All patients received at least one treatment break during chemoradiation. Each of the first 11 patients enrolled required at least one unscheduled treatment break as a result of the development of adverse events so the protocol was modified to allow an optional 9-day treatment break in the middle of chemoradiation. Among 17 patients treated with the modified protocol, 3 patients who did not take the optional break all required unscheduled breaks. Of the 14 patients who took the optional treatment break, 7 (50%) required a subsequent unscheduled break during chemoradiation.
Hospitalization was required for 3 patients (11%), all of whom completed therapy. One patient was admitted for vomiting and abdominal pain, one was admitted for dehydration, and one was admitted twice (once for a postoperative abdominal abscess and once for a rash and edema unrelated to therapy).
During chemoradiation, 25 patients (89%) experienced at least one grade 3 adverse event. Six (24%) of 25 patients experienced a grade 3 toxicity during chemotherapy. No grade 4 toxicities were reported. The specific grade 3 toxicities we observed are summarized in Table 2. The most common grade 3 hematologic complications were leukopenia (54%) and neutropenia (43%). The most common grade 3 nonhematologic complications were anorexia (29%), mucositis or stomatitis (29%), and fatigue (29%).
Recurrence and Survival
At the time of analysis, 19 patients (68%) had died; the median follow-up of the 9 surviving patients was 61.8 (range: 23.0–91.9) months. Among patients who died, 16 died with recurrent pancreatic cancer, 2 died of an unrelated cause at 18.9 months and 66.0 months, and 1 patient died at 29.4 months of unknown cause without a documented recurrence. The median OS of all 28 patients was 42 (95% confidence interval [CI] 32.1–64.5) months (Fig. 2a).
Twenty patients (71%) developed cancer recurrence during follow-up. The median DFS was 28.9 (95% CI 19.9–43.1) months (Supplementary Fig. 2b). An isolated local first recurrence was observed in 6 patients (21%). Isolated distant first recurrence was observed in 13 patients (46%) (lung, n = 5; liver, n = 6; peritoneum, n = 1; both the liver and spleen, n = 1). Combined local and distant (lung) first recurrence was observed in 1 patient.
Self-reported QOL
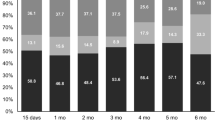
Mean self-reported scores of overall health and overall QOL at the midpoint of and upon completion of chemoradiation were poorer than at baseline (P < 0.001) (Fig. 3a, b). Moreover, the extent to which patients were troubled by the side effects of treatment was greater at both points than at baseline (P < 0.05) (Supplementary Fig. 3c). However, symptoms of depression during and immediately after chemoradiation were similar to those at baseline (P > 0.05) (Supplementary Fig. 3d).
Responses over time to specific questions in the QOL surveys. a “How would you rate your overall health during past week?” (C30). b “How would you rate your overall quality of life during past week?” (C30). Responses were converted to a discrete numerical scale; points represent mean (bars represent SD) of all patients’ responses to the query at each time point. Mid CXRT midpoint of chemoradiation, Post CXRT after chemoradiation, Pre CTX 1 before chemotherapy cycle 1, Pre CTX 2 before chemotherapy cycle 2; numbers refer to months after completion of therapy
Immediately before receipt of systemic 5-FU chemotherapy, mean scores of overall health, QOL, symptoms secondary to treatment, and depression were similar to those at baseline (P > 0.05). Between the chemotherapy cycles, treatment-related symptoms were greater than at baseline (P = 0.03), but symptoms of depression were lower than at baseline (P = 0.02).
Three months after completion of all therapy, mean scores for all questions were similar to baseline levels.
Prognostic Variables
Only lymph node status was significantly associated with OS (P = 0.008). Age, sex, performance status, surgical margin status, and the institution where surgery was performed were not associated with OS (P > 0.05).
Off-protocol Therapy
Our center has a preference for the administration of chemoradiation before surgery for resectable PDAC. During the time period in which this study was open, 56 patients not treated using this strategy underwent pancreaticoduodenectomy at M. D. Anderson as initial therapy for PDAC. Thirteen (23%) of these patients received adjuvant therapy on this protocol.
The median OS of the 43 patients who did not receive protocol therapy was 21.2 (95% CI 14.1–30.4) months. Fifteen (35%) of these patients did not receive any adjuvant therapy because of early cancer recurrence (n = 10), failure to regain a performance status sufficient for therapy (n = 4), or refusal (n = 1). The other 28 were scheduled to receive treatment outside of this trial. Nine of these 28 patients had an advanced age or performance status felt to preclude administration of interferon-based chemoradiation, 2 were declined an offer of protocol therapy, 7 wished to receive therapy at their referring institution, 2 did not meet eligibility criteria (other than performance status), 2 were offered systemic chemotherapy first because of clinical findings suggestive of but not diagnostic for recurrence, and 1 patient completed a similar chemoradiation program in the ACOSOG trial. The indications for delivery of adjuvant therapy outside of this trial were not recorded in 5 patients.
Eleven patients treated outside of this trial received systemic chemotherapy (typically gemcitabine-based) and chemoradiation (with concurrent 5-FU, capecitabine, or gemcitabine), 14 received systemic chemotherapy alone, and 1 received chemoradiation alone. The administration of therapy to the remaining 2 patients who were scheduled to begin adjuvant therapy could not be confirmed. The median OS of the 28 patients who were scheduled to receive adjuvant therapy outside of this trial was 23.7 (95% CI 18.9–37.8) months.
Discussion
In this phase II trial, the median OS of patients treated with interferon-based chemoradiation after macroscopically complete pancreaticoduodenectomy for PDAC was 42 months. Reversible grade 3 adverse events were observed in 89% of patients, but no grade 4 toxicity was reported. The negative impact of this aggressive chemoradiation regimen upon patients’ own perception of their overall health and QOL and upon their tolerance of treatment-related side effects was reversible upon completion of therapy.
Although this study was not randomized, these data add to the accumulating evidence of the activity and tolerability of adjuvant interferon-based chemoradiation in patients with potentially resectable PDAC (Table 3). The best overall outcomes were found in two single-institution experiences, including this one.6 The somewhat lower median OS reported in the third single-institution series may reflect the inclusion of patients with pancreatic body or tail cancers (20% of patients underwent distal or total pancreatectomy) and patients with stage III cancers (11%) in that study.14 Two multicenter experiences have also been completed. A single-arm phase II study coordinated by ACOSOG met its primary survival endpoint but closed just shy of its patient accrual target owing to concerns over toxicity.12 A larger, phase III study that randomized patients to receive interferon-based chemoradiation or systemic 5-FU could not demonstrate a significant statistical difference in OS between the two treatment arms (32.1 vs. 28.5 months).13 Nevertheless, the median OS of patients treated with interferon-based chemoradiation in that study is the highest ever reported in a multicenter trial of adjuvant therapy for PDAC.
Our data also confirm the substantial rate of adverse events that can be expected with this aggressive regimen in its current form (Table 3). However, in this study, as in the others, all adverse events were reversible, and no toxicity-related death was observed. Moreover, we found that a scheduled treatment break in the middle of chemoradiation was associated with a reduced need for unscheduled, toxicity-related treatment breaks. Finally—and perhaps most importantly, given the absence of serious long-term sequelae associated with these adverse events—we found that the negative effect of therapy upon patients’ overall QOL was transient and eliminated upon completion of therapy. Nevertheless, attempts to reduce the rates of toxicity of this regimen without affecting therapeutic efficacy should continue.
Given the toxicity profile of interferon-based chemoradiation, its administration should be limited to well-selected patients with excellent performance status. Enrollment in this study was limited to patients with a performance status of 0 or 1. Indeed, the regimen was delivered to only 23% of patients who underwent pancreaticoduodenectomy as initial therapy at our center during the enrollment period. Among the patients treated with alternative strategies outside of this trial, at least 32% had an age or performance status felt to preclude this aggressive approach. Nonetheless, 43% of patients treated off protocol completed alternate, rigorous chemoradiation programs. The extent to which patient selection influenced the relatively favorable OS seen among treated patients is therefore difficult to assess.
Moreover, despite their good performance status, enrolled patients had oncologically unfavorable pathologic factors: 100% had T3 tumors, 79% had positive lymph nodes, and their preoperative serum CA 19-9 levels (unadjusted for hyperbilirubinemia, when present) ranged from 3 to as high as 5557 U/dl. It is also important that, because it was performed at multiple centers, the primary surgical therapy administered to over half of the patients who were enrolled in this trial was neither standardized nor quality controlled. In a recent review of primary data sources associated with patients enrolled in the ACOSOG Z5031 trial, we found an overwhelming absence of consistency in perioperative staging, surgical treatment, and pathologic analysis among the patients reported to have undergone pancreaticoduodenectomy for resectable PDAC and that this inconsistency may have contributed to the high rate of local recurrence observed in that trial.21 The favorable OS reported here must be further appreciated in this context.
Finally, it must be emphasized that 35% of patients who were not enrolled in this trial but who underwent initial pancreaticoduodenectomy at our center during the same period never received postoperative therapy. This finding supports our continued bias toward neoadjuvant treatment sequencing for patients with potentially resectable PDAC.22 The favorable outcomes associated with postoperative interferon-based chemoradiation suggest the potential for a similar regimen to be effective in the preoperative setting. No reports of this approach exist, but favorable outcomes were observed when interferon-based chemoradiation was used as preoperative therapy in patients with esophageal cancer.23 This strategy might best be suited to patients with borderline resectable cancers, for whom a relatively poor prognosis would justify the choice of an aggressive regimen.
In summary, the data generated in this phase II study provide further support for the use of interferon-based chemoradiation regimens in the adjuvant setting for selected patients with PDAC.
References
Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903.
Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776–82.
Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77.
Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–26.
Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–71.
Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–80.
Ismail A, Van Groeningen CJ, Hardcastle A, et al. Modulation of fluorouracil cytotoxicity by interferon-alpha and -gamma. Mol Pharmacol. 1998;53:252–61.
Vokes EE. The promise of biochemical modulation in combined modality therapy. Semin Oncol. 1994;21:29–33.
Wadler S, Wersto R, Weinberg V, et al. Interaction of fluorouracil and interferon in human colon cancer cell lines: cytotoxic and cytokinetic effects. Cancer Res. 1990;50:5735–9.
Byfield JE, Calabro-Jones P, Klisak I, et al. Pharmacologic requirements for obtaining sensitization of human tumor cells in vitro to combined 5-fluorouracil or FTORAFUR and X rays. Int J Radiat Oncol Biol Phys. 1982;8:1923–33.
Holsti LR, Mattson K, Niiranen A, et al. Enhancement of radiation effects by alpha interferon in the treatment of small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1987;13:1161–6.
Picozzi VJ, Abrams RA, Decker PA, et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG trial Z05031. Ann Oncol. 2011;22:348–54.
Marten A, Schmidt J, Debus J, et al. CapRI: final results of the open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon-a2b (CRI) versus 5-FU alone for patients with resected pancreatic adenocarcinoma (PAC). J Clin Oncol. 2010;28(Suppl):LBA4012.
Linehan DC, Tan MC, Strasberg SM, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution phase II study. Ann Surg. 2008;248:145–51.
Common terminology criteria for adverse events (CTCAE), version 3. Washington, DC: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2003.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Hjermstad MJ, Fossa SD, Bjordal K, et al. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol. 1995;13:1249–54.
Fitzsimmons D, Johnson CD, George S, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939–41.
Radloff L. A new self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401.
Hwang RF, Wang H, Lara A, et al. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Ann Surg Oncol. 2008;15:1356–66.
Katz MH, Merchant NB, Brower S, et al. Standardization of surgical and pathologic variables is needed in multicenter trials of adjuvant therapy for pancreatic cancer: results from the ACOSOG Z5031 trial. Ann Surg Oncol. 2011;18:337–44.
Aloia TA, Lee JE, Vauthey JN, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–55.
Posner MC, Gooding WE, Landreneau RJ, et al. Preoperative chemoradiotherapy for carcinoma of the esophagus and gastroesophageal junction. Cancer J Sci Am. 1998;4:237–46.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1b
Treatment scheme after institution of an optional 9-day treatment break in the middle of chemoradiation (EPS 739 kb)
Supplementary Fig. 2b
Kaplan–Meier survival curve for disease-free survival (DFS) (EPS 729 kb)
Supplementary Fig. 3c
Responses over time to specific questions in the QOL surveys. c “To what extent have you been troubled by side effects from your treatment over past week?” (Quality of Life Questionnaire-Pancreas 26). Responses were converted to a discrete numerical scale; points represent mean (bars represent SD) of all patients’ responses to the query at each time point. Mid CXRT midpoint of chemoradiation, Post CXRT after chemoradiation, Pre CTX 1 before chemotherapy cycle 1; Pre CTX 2 before chemotherapy cycle 2. Numbers refer to months after completion of therapy (EPS 758 kb)
Supplementary Fig. 3d
Responses over time to specific questions in the QOL surveys. d “How many times have you felt sad over past week?” (Center for Epidemiologic Studies-Depression). Responses were converted to a discrete numerical scale; points represent mean (bars represent SD) of all patients’ responses to the query at each time point. Mid CXRT midpoint of chemoradiation, Post CXRT after chemoradiation, Pre CTX 1 before chemotherapy cycle 1; Pre CTX 2 before chemotherapy cycle 2. Numbers refer to months after completion of therapy (EPS 757 kb)
Rights and permissions
About this article
Cite this article
Katz, M.H.G., Wolff, R., Crane, C.H. et al. Survival and Quality of Life of Patients with Resected Pancreatic Adenocarcinoma Treated with Adjuvant Interferon-Based Chemoradiation: A Phase II Trial. Ann Surg Oncol 18, 3615–3622 (2011). https://doi.org/10.1245/s10434-011-1847-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1847-4