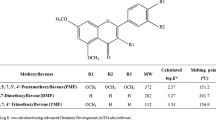
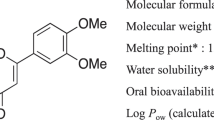
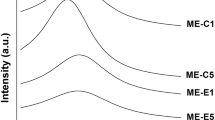
Abstract
Curcuma comosa (C. comosa) is widely used in traditional medicine as a dietary supplement for health promotion in postmenopausal women in Thailand. It contains several diarylheptanoids, which are considered to be a novel class of phytoestrogens. However, the diarylheptanoids isolated from the plant rhizome are shown to have low oral bioavailability and faster elimination characteristics. The aim of this study was to investigate the permeation behavior of the active compounds of diarylheptanoids. The effects of binary vehicle systems and permeation enhancers on diarylheptanoids permeation and accumulation within the skin were studied using side-by-side diffusion cells through the porcine ear skin. Among the tested binary vehicle systems, the ethanol/water vehicle appeared to be the most effective system for diarylheptanoids permeation with the highest flux and shortest lag time. The presence of transcutol in the vehicle system significantly increased diarylheptanoid’s permeation and accumulation within the skin in a concentration-dependent manner. Although the presence of terpenes in formulation decreased the flux of diarylheptanoids, it raised the amount of diarylheptanoids retained within the skin substantially. Based on the feasibility of diarylheptanoid permeation, C. comosa extract should be further developed into an effective transdermal product for health benefits and hormone replacement therapy.




Similar content being viewed by others
Abbreviations
- C. comosa :
-
Curcuma comosa
- D:
-
Diffusion coefficient
- DA:
-
Diarylheptanoids
- HPLC:
-
High-performance liquid chromatography
- K:
-
Partition coefficient
- P:
-
Permeability coefficient
- PBS:
-
Phosphate buffer saline
- PG:
-
Propylene glycol
- PEG:
-
Polyethylene glycol
- TEWL:
-
Transepidermal water loss
- Tlag :
-
Lag time
References
Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19(4):397–428.
Winuthayanon W, Piyachaturawat P, Suksamrarn A, Ponglikitmongkol M, Arao Y, Hewitt SC, et al. Diarylheptanoid phytoestrogens isolated from the medicinal plant Curcuma comosa: biologic actions in vitro and in vivo indicate estrogen receptor-dependent mechanisms. Environ Health Perspect. 2009;117(7):1155–61.
Piyachaturawat P, Ercharuporn S, Suksamrarn A. Uterotrophic effect of Curcuma comosa in rats. Int J Pharmacogn. 1995;33(4):5.
Winuthayanon W, Suksen K, Boonchird C, Chuncharunee A, Ponglikitmongkol M, Suksamrarn A, et al. Estrogenic activity of diarylheptanoids from Curcuma comosa Roxb. requires metabolic activation. J Agric Food Chem. 2009;57(3):840–5.
Sornkaewa N, Lin Y, Wang F, Zhang G, Chokchaisiri R, Zhang A, et al. Diarylheptanoids of Curcuma comosa with inhibitory effects on nitric oxide production in macrophage RAW 264.7 cells. Nat Prod Commun. 2015;10(1):89–93.
Sodsai A, Piyachaturawat P, Sophasan S, Suksamrarn A, Vongsakul M. Suppression by Curcuma comosa Roxb. of pro-inflammatory cytokine secretion in phorbol-12-myristate-13-acetate stimulated human mononuclear cells. Int Immunopharmacol. 2007;7(4):524–31.
Tantikanlayaporn D, Wichit P, Weerachayaphorn J, Chairoungdua A, Chuncharunee A, Suksamrarn A, et al. Bone sparing effect of a novel phytoestrogen diarylheptanoid from Curcuma comosa Roxb. in ovariectomized rats. PloS one. 2013;8(11):e78739.
Weerachayaphorn J, Chuncharunee A, Mahagita C, Lewchalermwongse B, Suksamrarn A, Piyachaturawat P. A protective effect of Curcuma comosa Roxb. on bone loss in estrogen deficient mice. J Ethnopharmacol. 2011;137(2):956–62.
Piyachaturawat P, Teeratagolpisal N, Toskulkao C, Suksamrarn A. Hypolipidemic effect of Curcuma comosa in mice. Artery. 1997;22(5):233–41.
Su J, Sripanidkulchai B, Sripanidkulchai K, Piyachaturawat P, Wara-Aswapati N. Effect of Curcuma comosa and estradiol on the spatial memory and hippocampal estrogen receptor in the post-training ovariectomized rats. J Nat Med. 2011;65(1):57–62.
Su J, Sripanidkulchai K, Wyss JM, Sripanidkulchai B. Curcuma comosa improves learning and memory function on ovariectomized rats in a long-term Morris water maze test. J Ethnopharmacol. 2010;130(1):70–5.
Su J, Sripanidkulchai K, Hu Y, Chaiittianan R, Sripanidkulchai B. Increased in situ intestinal absorption of phytoestrogenic diarylheptanoids from Curcuma comosa in nanoemulsions. AAPS PharmSciTech. 2013;14(3):1055–62.
Su J, Sripanidkulchai K, Suksamrarn A, Hu Y, Piyachuturawat P, Sripanidkulchai B. Pharmacokinetics and organ distribution of diarylheptanoid phytoestrogens from Curcuma comosa in rats. J Nat Med. 2012;66(3):468–75.
Hamishehkar H, Khoshbakht M, Jouyban A, Ghanbarzadeh S. The relationship between solubility and transdermal absorption of tadalafil. Adv Pharm Bull. 2015;5(3):411–7.
Suwanpidokkul N, Thongnopnua P, Umprayn K. Transdermal delivery of zidovudine (AZT): the effects of vehicles, enhancers, and polymer membranes on permeation across cadaver pig skin. AAPS PharmSciTech. 2004;5(3), e48.
Sullivan Jr DW, Gad SC, Julien M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem Toxicol. 2014;72:40–50.
Mura P, Faucci MT, Bramanti G, Corti P. Evaluation of transcutol as a clonazepam transdermal permeation enhancer from hydrophilic gel formulations. Eur J Pharm Sci. 2000;9(4):365–72.
Sapra B, Jain S, Tiwary AK. Percutaneous permeation enhancement by terpenes: mechanistic view. AAPS J. 2008;10(1):120–32.
Vaddi HK, Ho PC, Chan YW, Chan SY. Terpenes in ethanol: haloperidol permeation and partition through human skin and stratum corneum changes. J Control Release. 2002;81(1–2):121–33.
Gao S, Singh J. In vitro percutaneous absorption enhancement of a lipophilic drug tamoxifen by terpenes. J Control Release. 1998;51(2–3):193–9.
Narishetty ST, Panchagnula R. Transdermal delivery of zidovudine: effect of terpenes and their mechanism of action. J Control Release. 2004;95(3):367–79.
Machado M, Salgado TM, Hadgraft J, Lane ME. The relationship between transepidermal water loss and skin permeability. Int J Pharm. 2010;384(1–2):73–7.
Smith HR, Rowson M, Basketter DA, McFadden JP. Intra-individual variation of irritant threshold and relationship to transepidermal water loss measurement of skin irritation. Contact Dermatitis. 2004;51(1):26–9.
Gwak HS, Chun IK. Effect of vehicles and penetration enhancers on the in vitro percutaneous absorption of tenoxicam through hairless mouse skin. Int J Pharm. 2002;236(1–2):57–64.
Lee PJ, Ahmad N, Langer R, Mitragotri S, Prasad SV. Evaluation of chemical enhancers in the transdermal delivery of lidocaine. Int J Pharm. 2006;308(1–2):33–9.
Tuntiyasawasdikul S, Limpongsa E, Jaipakdee N, Sripanidkulchai B. Transdermal permeation of Kaempferia parviflora methoxyflavones from isopropyl myristate-based vehicles. AAPS PharmSciTech. 2014;15(4):947–55.
Aungst BJ, Blake JA, Hussain MA. Contributions of drug solubilization, partitioning, barrier disruption, and solvent permeation to the enhancement of skin permeation of various compounds with fatty acids and amines. Pharm Res. 1990;7(7):712–8.
Panchagnula R, Salve PS, Thomas NS, Jain AK, Ramarao P. Transdermal delivery of naloxone: effect of water, propylene glycol, ethanol and their binary combinations on permeation through rat skin. Int J Pharm. 2001;219(1–2):95–105.
Oh HJ, Oh YK, Kim CK. Effects of vehicles and enhancers on transdermal delivery of melatonin. Int J Pharm. 2001;212(1):63–71.
Lane ME. Skin penetration enhancers. Int J Pharm. 2013;447(1–2):12–21.
Wen H, Hao JS, Li SK. Influence of permeant lipophilicity on permeation across human sclera. Pharm Res-Dordr. 2010;27(11):2446–56.
Rao Y, Zheng F, Zhang X, Gao J, Liang W. In vitro percutaneous permeation and skin accumulation of finasteride using vesicular ethosomal carriers. AAPS PharmSciTech. 2008;9(3):860–5.
Godwin DA, Kim NH, Felton LA. Influence of Transcutol CG on the skin accumulation and transdermal permeation of ultraviolet absorbers. Eur J Pharm Biopharm. 2002;53(1):23–7.
El Maghraby GM, Alanazi FK, Alsarra IA. Transdermal delivery of tadalafil. I. Effect of vehicles on skin permeation. Drug Dev Ind Pharm. 2009;35(3):329–36.
Panchagnula R, Ritschel WA. Development and evaluation of an intracutaneous depot formulation of corticosteroids using Transcutol as a cosolvent: in-vitro, ex-vivo and in-vivo rat studies. J Pharm Pharmacol. 1991;43(9):609–14.
Williams AC, Barry BW. Terpenes and the lipid-protein-partitioning theory of skin penetration enhancement. Pharm Res. 1991;8(1):17–24.
Fang JY, Hung CF, Chiu HC, Wang JJ, Chan TF. Efficacy and irritancy of enhancers on the in-vitro and in-vivo percutaneous absorption of curcumin. J Pharm Pharmacol. 2003;55(8):1175.
Okabe H, Obata Y, Takayama K, Nagai T. Percutaneous absorption enhancing effect and skin irritation of monocyclic monoterpenes. Drug Des Deliv. 1990;6(3):229–38.
Songkro S, Rades T, Becket G. Effects of some terpenes on the in vitro permeation of LHRH through newborn pig skin. Pharmazie. 2009;64(2):110–5.
Acknowledgments
The research was financially supported by the Center for Research and Development of Herbal Health Products, Faculty of Pharmaceutical Sciences, and received scholarship under the Post-doctoral Program from Research Affairs and Graduate School, Khon Kaen University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tuntiyasawasdikul, S., Limpongsa, E., Jaipakdee, N. et al. Effects of Vehicles and Enhancers on the Skin Permeation of Phytoestrogenic Diarylheptanoids from Curcuma comosa . AAPS PharmSciTech 18, 895–903 (2017). https://doi.org/10.1208/s12249-016-0582-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0582-3