Abstract
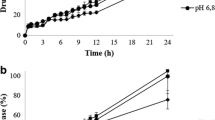
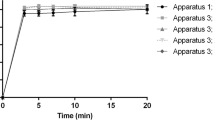
This article provides an analysis of dissolution kinetics associated with formulations subjected to different dissolution methods with the purpose of revealing credible direction on selection of apparatus type and hydrodynamics on in vitro drug release profiles using three different formulations. The dissolution kinetics of immediate release (IR) and controlled release (CR) ibuprofen tablets under different hydrodynamic conditions were determined, and potential existence of any correlation between USP apparatus I and II were analyzed using adequate kinetic models. Two types of CR tablets based on PEO (polyethylene oxide-N80) and HPMC (hydroxypropyl methylcellulose- K100M) polymers were prepared. Marketed ibuprofen 200-mg IR tablets were also used. Dissolution studies were carried out using USP 34 apparatuses I and II methods at stirring speed of 100 and 50 rpm in 900 mL phosphate buffer, pH 7.2 at 37°C. The drug release profiles for each formulation was determined and statistically analyzed using model-dependent, model-independent (f 2 ), and ANOVA methods. No significant dissolution differences existed between IR tablets, whereas CR tablets were significantly impacted by apparatus types and hydrodynamics. PEO matrices displayed higher sensitivity to hydrodynamics relative to HPMC matrices, and differences in dissolution profiles were confirmed by ANOVA and boxplot analysis. It is concluded that in the case of CR systems, selection of apparatus type and adherence to the monograph specifications and hydrodynamic conditions is critical, while for IR tablets, both apparatus types and agitation rates had no significant impact on drug release rate, suggesting the possibility of apparatus interchangeability if desired.






Similar content being viewed by others
REFERENCES
Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–4.
Brunner E. Reaktionsgeschwindigkeit in heterogenen Systemen. Z Phys Chem. 1904;43:56–102.
Nernst W. Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Z Phys Chem. 1904;47:52–5.
Macheras P, Dokoumetzidis A. On the heterogeneity of drug dissolution and release. Pharm Res. 2000;17(2):108–12.
Weibull W. A statistical distribution function of wide applicability. J Appl Mech. 1951;18:293–7.
Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. J Pharm Pharmacol. 1972;24:979–81.
Costa P, Lobo JS. Influence of dissolution medium agitation on release profiles of sustained-release tablets. Drug Dev Ind Pharm. 2001;27(8):811–7.
Missaghi S, Fassihi R. Release characterization of dimenhydrinate from an eroding and swelling matrix: selection of appropriate dissolution apparatus. Int J Pharm. 2005;293(1):35–42.
Pillay V, Fassihi R. Unconventional dissolution methodologies. J Pharm Sci. 1999;88(9):843–51.
Underwood FL, Cadwallader DE. Effects of various hydrodynamic conditions on dissolution rate determinations. J Pharm Sci. 1976;65(5):697–700.
Shah VP, Gurbarg M, Noory A, Dighe S, Skelly JP. Influence of higher rates of agitation on release patterns of immediate-release drug products. J Pharm Sci. 1992;81(6):500–3.
Jamzad S, Fassihi R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—a technical note. AAPS PharmScitech. 2006;7(2):E17–22.
Pillay V, Fassihi R. A new method for dissolution studies of lipid-filled capsules employing nifedipine as a model drug. Pharm Res. 1999;16(2):333–7.
Mirza T, Joshi Y, Liu Q, Vivilecchia R. Evaluation of dissolution hydrodynamics in the USP, Peak™ and flat-bottom vessels using different solubility drugs. Dissolution Technol. 2005;12(1):11–6.
Röst M, Quist PO. Dissolution of USP prednisone calibrator tablets: effects of stirring conditions and particle size distribution. J Pharm Biomed Anal. 2003;31(6):1129–43.
Wurster DE, Taylor PW. Dissolution rates. J Pharm Sci. 1965;54(2):169–75.
Levy G. Effect of particle size on dissolution and gastrointestinal absorption rates of pharmaceuticals. Am J Pharm Sci Support Public Health. 1963;135:78–92.
Shaw LR, Irwin WJ, Grattan TJ, Conway BR. The effect of selected water-soluble excipients on the dissolution of paracetamol and ibuprofen. Drug Dev Ind Pharm. 2005;31(6):515–25.
Yuksel N, Kanık AE, Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and-independent methods. Int J Pharm. 2000;209(1):57–67.
Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1.
Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9.
Varelas CG, Dixon DG, Steiner C. Zero-order release from biphasic polymer hydrogels. J Control Release. 1995;34:185–92.
Mulye NV, Turco SJ. A simple model based on first order kinetics to explain release of highly water soluble drugs from porous dicalcium phosphate dihydrate matrices. Drug Dev Ind Pharm. 1995;21:943–53.
Baker RW, Lonsdale HS. Controlled release: mechanisms and rates. In: Taquary AC, Lacey RE, editors. Controlled release of biologically active agents. New York: Plenum Press; 1974. p. 15–71.
Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23:923–31.
Goldsmith JA, Randall N, Ross SD. On methods of expressing dissolution rate data. J Pharm Pharmacol. 1978;30:347–9.
Hanson W. Handbook of dissolution testing. 2nd ed. Eugene Oregon: Aster Publishing Corporation; 1991. p. 35.
Guidance for Industry: Immediate Release Solid Oral Dosage Forms Scale-up and Postapproval Changes: Chemistry, Manufacturing, and Controls. In Vitro Dissolution Testing and in Vivo Bioequivalence Documentation. Center for Drug Evaluation and Research (CDER), CMC 5. 1995. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM070636.pdf. Accessed Nov 1995.
Gonçalves-Araújo T, Rajabi-Siahboomi AR, Caraballo I. Polymer percolation threshold in HPMC extended release formulation of carbamazepine and verapamil HCl. AAPS PharmSciTech. 2010;11(2):558–62.
Berens AR, Hopfenberg HB. Diffusion and relaxation in glassy polymer powders: 2. Separation of diffusion and relaxation parameters. Polymer. 1978;19(5):489–96.
Dürig T, Fassihi R. Guar-based monolithic matrix systems: effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics. J Control Release. 2002;80(1):45–56.
Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm. 1989;57(2):169–72.
Pham AT, Lee PI. Probing the mechanisms of drug release from hydroxypropylmethyl cellulose matrices. Pharm Res. 1994;11(10):1379–84.
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
USP, General Chapter “ < 711 > Dissolution” USP 34-NF 29. Rockville: The United States Pharmacopeial Convention, 2011. p. 278–284.
USP, General Chapter “ < 724 > Drug release” USP 34-NF 29. Rockville: The United States Pharmacopeial Convention, 2011. p. 285–289.
USP, General Chapter “ < 1088 > In vitro and in vivo evaluation of dosage forms” USP 34-NF 29. Rockville: The United States Pharmacopeial Convention, 2011. p. 612–617.
USP, General Chapter “ < 1225 > Validation of compendial procedures” USP 34-NF 29. Rockville: The United States Pharmacopeial Convention, 2011. p. 778–782.
Guidance for Industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2015. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070246.pdf. Accessed May 2015.
Note for guidance on the investigation of bioavailability and bioequivalence. Committee for Proprietary Medicinal Products (CPMP). 2000. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003519.pdf. Accessed 14 Dec 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Z., Fassihi, R. Mechanistic Approach to Understanding the Influence of USP Apparatus I and II on Dissolution Kinetics of Tablets with Different Operating Release Mechanisms. AAPS PharmSciTech 18, 462–472 (2017). https://doi.org/10.1208/s12249-016-0535-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0535-x