Abstract
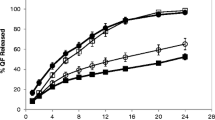
It is challenging to achieve mechanically robust drug-release profiles from hydrophilic matrices containing a high dose of a drug with good solubility. However, a mechanically robust drug release over prolonged period of time can be achieved, especially if the viscosity and amount of the polymer is sufficiently high, above the “threshold values.” The goal of this research was to determine the hydroxypropyl cellulose (HPC) and hydroxypropyl methylcellulose (HPMC) polymer threshold amount that would enable robust drug release from matrix tablets containing a high dose of levetiracetam as a class I model drug according to the Biopharmaceutical Classification System (BCS). For this purpose, formulations containing HPC or HPMC of similar viscosity range, but in different amounts, were prepared. Based on the dissolution results, two final formulations were selected for additional in vitro and in vivo evaluation to confirm the robustness and to show bioequivalence. Tablets were exposed to various stress conditions in vitro with the use of different mechanically stress-inducing dissolution methods. The in vitro results were compared with in vivo results obtained from fasted and fed bioequivalence studies. Under both conditions, the formulations were bioequivalent and food had a negligible influence on the pharmacokinetic parameters C max and area under the curve (AUC). It was concluded that the drug release from both selected formulations is mechanically robust and that HPC and HPMC polymers with intrinsic viscosities above 9 dL/g and in quantities above 30% enable good mechanical resistance, which ensures bioequivalence. In addition, HPC matrices were found to be more mechanically robust compared to HPMC.




Similar content being viewed by others

References
Liu P, Ju T, Qiu Y. Diffusion-controlled drug delivery systems. In: Li X, Jasti BR, editors. Design of controlled release drug delivery systems. United States: McGraw-Hill; 2006. p. 107–37.
Wang Z, Shmeis RA. Dissolution controlled drug delivery systems. In: Li X, Jasti BR, editors. Design of controlled release drug delivery systems. United States: McGraw-Hill; 2006. p. 139–72.
Siepmann J, Peppas NA. Hydrophilic matrices for controlled drug delivery: an improved mathematical model to predict the resulting drug release kinetics (the “Sequential Layer” model). Pharm Res. 2000;17(10):1290–8.
Baumgartner S, Kristl J, Peppas N. Network structure of cellulose ethers used in pharmaceutical applications during swelling and at equilibrium. Pharm Res. 2002;19:1084–90.
Baumgartner S, Lahajnar G, Sepe A, Kristl J. Investigation of the state and dynamics of water in hydrogels of cellulose ethers by 1H NMR spectroscopy. AAPS Pharm Sci Technol. 2002;3(4):E36.
McConnell EL, Fadda HM, Basit AW. Gut instincts: explorations in intestinal physiology and drug delivery. Int J Pharm. 2008;364:213–26.
Weitschies W, Blume H, Mönnikes H. Magnetic marker monitoring: high resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur J Pharm Biopharm. 2010;74:93–101.
Goodman K, Hodges LA, Band J, Stevens HNE, Weitschies W, Wilson CG. Assessing gastrointestinal motility and disintegration profiles of magnetic tablets by a novel magnetic imaging device and gamma scintigraphy. Eur J Pharm Biopharm. 2010;74:84–92.
Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharm. 2010;7(5):1388–405.
Abrahamsson B, Albery T, Eriksson A, Gustafsson I, Sjoberg M. Food effects on tablet disintegration. Eur J Pharm Sci. 2004;22:165–72.
Abrahamsson B, Pal A, Sjoberg M, Carlsson M, Laurell E, Brasseur JG. A novel in vitro and numerical analysis of shear-induced drug release from extended-release tablets in the fed stomach. Pharm Res. 2005;22(8):1215–26. doi:10.1007/s11095-005-5272-x.
Dokoumetzidis A, Macheras P. IVIVC of controlled release formulations: physiological–dynamical reasons for their failure. J Control Release. 2008;129:76–8.
Fotaki N, Aivaliotis A, Butler J, Dressman JB, Fischbach M, Hempenstall J, et al. A comparative study of different release apparatus in generating in vitro-in vivo correlations for extended release formulations. Eur J Pharm Biopharm. 2009;73:115–20.
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2013. doi:10.1016/j.ejps.2013.08.024.
Sako K, Sawada T, Nakashima H, Yokohama S, Sonobe T. Influence of water soluble fillers in hydroxypropylmethylcellulose matrices on in vitro and in vivo drug release. J Control Release. 2002;81:165–72.
Garbacz G, Wedemeyer R, Nagel S, Giessmann T, Mönnike H, Wilson CG, et al. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. Eur J Pharm Biopharm. 2008;70:421–8.
Aoki S, Ando H, Tatsuishi K, Uesugi K, Ozawa H. Determination of the mechanical impact force in the in vitro dissolution test and evaluation of the correlation between in vivo and in vitro release. Int J Pharm. 1993;95:67–75.
Rohrs B, Burch-Clark DL, Witt MJ, Stelzer DJ. USP dissolution apparatus 3 (reciprocating cylinder): instrument parameter effects on drug release from sustained-release formulations. J Pharm Sci. 1995;84(8):922–6.
Mu X, Tobyn MJ, Staniforth JN. Development and evaluation of bio-dissolution systems capable of detecting the food effect on a polysaccharide-based matrix system. J Control Release. 2003;93:309–18.
Klein S, Rudolph MW, Skalsky B, Petereit HU, Dressman JB. Use of the BioDis to generate a physiologically relevant IVIVC. J Control Release. 2008;130:216–9.
Klančar U, Horvat M, Baumgartner S. Correlating cellulose derivative intrinsic viscosity with mechanical susceptibility of swollen hydrophilic matrix tablets. AAPS Pharm Sci Technol. 2012;13(3):903–10.
Klančar U, Markun B, Legen I, Baumgartner S. A novel beads-based dissolution method for the in vitro evaluation of extended release HPMC matrix tablets and the correlation with the in vivo data. AAPS J. 2013;15(1):267–77.
Maderuelo C, Zarzuelo A, Lanao Usala JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J Control Release. 2011;154:2–19.
Dow Chemical Company. Using METHOCEL cellulose ethers for controlled release of drugs in hydrophilic matrix systems. DOW, July 2002.
Anand O, Yu LX, Conner DP, Davit BM. Dissolution testing for generic drugs: an FDA perspective. AAPS J. 2011;13(3):328–35.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35.
Castellanos Gil E, Iraizoz Colarte A, Bataille B, Caraballo I. Estimation of the percolation thresholds in Lobenzarit disodium native dextran matrix tablets. AAPS Pharm Sci Technol. 2007;8(4):E115.
Barakat NS, Elbagory IM, Almurshedi AS. Controlled-release carbamazepine matrix granules and tablets comprising lipophilic and hydrophilic components. Drug Deliv. 2009;16(1):57–65.
Walden M, Nicholls FA, Smith KJ. The effect of ethanol on the release of opioids from oral prolonged-release preparations. Drug Dev Ind Pharm. 2007;33:1101–11.
Tucker GT, Emeje MO, Nwabunike PI, Isimi CY, Kunle OO, Ofoefule SI. Hydro-alcoholic media: an emerging in vitro tool for predicting dose dumping from controlled release matrices. J Pharmacol Toxicol. 2008;3(2):84–92.
Sarid-Segal O, Piechniczek-Buczek J, Knapp C, Afshar M, Devine E, Sickles L, et al. The effects of levetiracetam on alcohol consumption in alcohol-dependent subjects: an open label study. Am J Drug Alcohol Abuse. 2008;34:441–7.
Lowman AM, Peppas NA. Hydrogels. In: Mathiowitz E, editor. Encyclopedia of controlled drug delivery. New York: Wiley; 2000. p. 397–4.
EMA. Guideline on quality of oral modified release products 2012; EMA/492713/2012.
FDA. Guidance for industry, extended release oral dosage forms: development, evaluation and application of an in vitro/in vivo correlation. 1997; FDA, CDER.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klančar, U., Baumgartner, S., Legen, I. et al. Determining the Polymer Threshold Amount for Achieving Robust Drug Release from HPMC and HPC Matrix Tablets Containing a High-Dose BCS Class I Model Drug: In Vitro and In Vivo Studies. AAPS PharmSciTech 16, 398–406 (2015). https://doi.org/10.1208/s12249-014-0234-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0234-4