Abstract
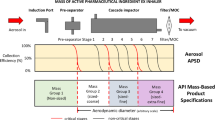
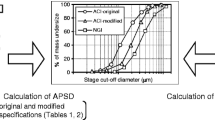
The multi-stage cascade impactor (CI) is widely used to determine aerodynamic particle size distributions (APSDs) of orally inhaled products. Its size-fractionating capability depends primarily on the size of nozzles of each stage. Good Cascade Impactor Practice (GCIP) requires that these critical dimensions are linked to the accuracy of the APSD measurement based on the aerodynamic diameter size scale. Effective diameter (D eff) is the critical dimension describing any nozzle array, as it is directly related to stage cut-point size (d 50). d 50 can in turn be determined by calibration using particles of known aerodynamic diameter, providing traceability to the international length standard. Movements in D eff within manufacturer tolerances for compendial CIs result in the worst case in shifts in d 50 of <±10%. Stage mensuration therefore provides satisfactory control of measurement accuracy. The accurate relationship of D eff to d 50 requires the CI system to be leak-free, which can be checked by sealing the apparatus at the entry to the induction port and isolating it from the vacuum source and measuring the rate of pressure rise before each use. Mensuration takes place on an infrequent basis compared with the typical interval between individual APSD determinations. Measurement of stage flow resistance (pressure drop; ΔP stage) could enable the user to know that the CI stages are fit for use before every APSD measurement, by yielding an accurate measure of D eff. However, more data are needed to assess the effects of wear and blockage before this approach can be advocated as part of GCIP.





Similar content being viewed by others
References
Christopher D, Curry P, Doub B, Furnkranz K, Lavery M, Lin K, et al. Considerations for the development and practice of cascade impaction testing including a mass balance failure investigation tree. J Aerosol Med. 2003;16(3):235–47.
Mitchell J, Newman S, Chan H-K. In vitro and in vivo aspects of cascade impactor tests and inhaler performance: a review. AAPS PharmSciTech. 2007;8(4). doi:10.1208/pt0804110. Article 110.
Newman SP, Chan H-K. In vitro/in vivo comparisons in pulmonary drug delivery. J Aerosol Med. 2008;21(1):1–8.
European Directorate for Quality in Medicines and HealthCare (EDQM). Monograph 2.9.18: European Pharmacopeia, Preparations for inhalation: aerodynamic assessment of fine particles, Council of Europe, 67075 Strasbourg, France, 2012; Ph.Eur.7.5 [USB 7th ed. (7.52012)].
United States Pharmacopeial Convention. Chapter 601: Aerosols, Nasal Sprays, Metered-Dose Inhalers and Dry Powder Inhalers. Rockville, MD, USA, 2012; USP 35-NF 30.
European Medicines Agency (EMA). Guideline on the Pharmaceutical Quality of Inhalation and Nasal Products London, UK, 2006; EMEA/CHMP/QWP/49313/2005 Corr. 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003568.pdf. Accessed 4 Oct 2012.
Health Canada (HC): Guidance for Industry: Pharmaceutical Quality of Inhalation and Nasal Products, Ottawa, Canada, 2006; Document 06-106624-547. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/chem/inhalationnas-eng.php. Accessed 4 Oct 2012
United States Food and Drug Administration (FDA). Draft Guidance: Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products Chemistry, Manufacturing and Controls Documentation. United States Food and Drug Administration, Rockville, MD, USA. 1998; Docket 98D-0997. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070573.pdf. Accessed 4 Oct 2012.
Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2003;16(4):341–77.
Mitchell JP. Particle standards: their development and application. Kona Powder Part J. 2000;18:41–59.
Mitchell JP, Nagel MW, Doyle C, Ali RS, Avvakoumova V, Christopher D, Quiroz J, Strickland H, Tougas T, Lyapustina S. Relative precision of inhaler aerodynamic particle size distribution (APSD) metrics by full resolution and abbreviated Andersen Cascade Impactors (ACIs): part 1. AAPS PharmSciTech. 2010;11(2):843–51.
Mitchell JP, Nagel MW, Doyle C, Ali RS, Avvakoumova V, Christopher D, Quiroz J, Strickland H, Tougas T, Lyapustina S. Relative precision of inhaler aerodynamic particle size distribution (APSD) metrics by full resolution and abbreviated Andersen Cascade Impactors (ACIs): part 2—investigation of bias in extra-fine mass fraction with AIM-HRT impactor. AAPS PharmSciTech. 2010;11(3):1115–8.
Mitchell JP, Tougas T, Christopher JD, Lyapustina S, Glaab V. The Abbreviated Impactor Measurement and Efficient Data Analysis concepts: why use them and when. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory drug delivery—2012. River Grove: Davis Healthcare; 2012. p. 731–5.
European Directorate for Quality in Medicines and HealthCare (EDQM). Monograph 2.9.44: European Pharmacopeia, Preparations for nebulisation: characterisation. Council of Europe, 67075 Strasbourg, France, 2012; Ph.Eur.7.5 [USB 7th ed. (7.52012)].
United States Pharmacopeial Convention. Chapter 1601: Products for Nebulization—Characterization Tests. Rockville, MD, USA, 2012; USP 35-NF 30.
Dolovich M, Rhem R. Impact of oropharyngeal deposition on inhaled dose. J Aerosol Med. 1998;11(Supple 1):112–5.
Mitchell JP, Copley M, Sizer Y, Russell T, Solomon D. Adapting the Abbreviated Impactor Measurement (AIM) concept to make appropriate inhaler aerosol measurements to compare with clinical data: a scoping study with the “Alberta” Idealized Throat (AIT) inlet. J Aerosol Med Pulmon Deliv. 2012;25(4):188–97.
Zhang Y, Gilbertson K, Finlay WH. In vivo–in vitro comparison of deposition in three mouth–throat models with Qvar® and Turbuhaler® inhalers. J Aerosol Med. 2007;20(3):227–35.
Stein SW, Gabrio BJ. Understanding throat deposition during cascade impactor testing. In: Dalby RN, Byron PR, Farr SJ, Peart J, editors. Respiratory drug delivery VII. Raleigh: Serentec; 2000. p. 573–6.
Marple VA, Olson BA, Santhanakrishnan K, Roberts DL, Mitchell JP, Hudson-Curtis BL. Next generation pharmaceutical impactor. Part II. Calibration. J Aerosol Med. 2003;16(3):301–24.
Tong Z, Yang R, Yu A, Adi S, Chan H-K. Unravelling the mechanics of deagglomeration through experiments based upon complex modelling theory. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory drug delivery—2012. River Grove: Davis Healthcare; 2012. p. 355–65.
Tong ZB, Yang RY, Yu AB, Chan H-K. Numerical study of aerosolization of carrier based dry powder inhalation systems. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory drug delivery—2012. River Grove: Davis Healthcare; 2012. p. 821–6.
Dunbar C, Kataya A, Tiangbe T. Reducing bounce effects in the Andersen cascade impactor. Int J Pharm. 2005;301:25–32.
Copley M, Mitchell J, Solomon D. Evaluating the Alberta throat: an innovation to support the acquisition of more clinically applicable aerosol aerodynamic particle size distribution (APSD) data in oral inhaled product (OIP) development. Inhal. 2011;5(4):12–6.
Zhang Y, Finlay WH. Experimental measurements of particle deposition in three proximal lung bifurcation models with an idealized mouth–throat. J Aerosol Med. 2005;18(4):460–73.
Roberts DL, Marple VA. Induction port assembly for impactors and method for assembly. US Pat. Appl. Pub. 2002/0070548, June 13, 2002.
Moody LF. Friction factors for pipe flows. Trans ASME. 1944;66(8):671–84.
Marple VA, Roberts DL, Romay FJ, Miller NC, Truman KG, Van Oort M, Olsson B, Holroyd MJ, Mitchell JP, Hochrainer D. Next generation pharmaceutical impactor. Part 1: design. J Aerosol Med. 2003;16(3):283–99.
Sethuraman VV, Hickey AJ. Evaluation of pre-separator performance for the 8-stage nonviable Andersen impactor. AAPS PharmSciTech. 2001;2(1):E4. doi:10.1208/pt020104.
Copley Scientific Ltd. Quality solutions for inhaler testing—brochure. Nottingham, UK: Copley Scientific Limited; 2012, p.35. Available at: http://www.copleyscientific.com/editorials.asp?c=42&d=13, Accessed 10 Aug 2012.
Marple VA, Liu BYH. Characteristics of laminar jet impactors. Environ Sci Technol. 1974;8(7):648–54.
Andersen A. A sampler for respiratory health assessment. Am Ind Hyg Assoc J. 1966;27(2):160–5.
Boundy MG, Leith D, Knowles MR. Dispersion and size distribution of amiloride by metered dose and dry powder inhalers. J Aerosol Med. 1990;3(4):233–41.
Smurthwaite M. Westech Instrument Services, UK. Personal communication. 2011.
Roberts DL. Theory of multi-nozzle impactor stages and the interpretation of stage mensuration data. Aerosol Sci Technol. 2009;43(11):1119–29.
Stein S, Olson BA. Variability in size distribution measurements obtained using multiple Andersen mark II cascade impactors. Pharm Res. 1997;14(12):1718–25.
Nichols SC. Calibration and mensuration issues for the standard and modified impactor. Pharmeuropa. 2000;12(4):585.
Nichols SC. Particle size distribution parameters using the Next Generation pharmaceutical impactor. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory drug delivery—IX. River Grove: Davis Healthcare; 2004. p. 485–7.
Asking L, Olsson B. Calibration at different flow rates of a multistage liquid impinger. Aerosol Sci Technol. 1997;27(1):39–49.
Marple VA, Olson BA, Santhanakrishnan K, Roberts DL, Mitchell JP, Hudson-Curtis BL. Next generation pharmaceutical impactor. Part III. Extension of archival calibration to 15 L/min. J Aerosol Med. 2004;17(4):335–43.
Roberts DL. MSP Corporation. Personal communication. 2012.
Olsson B, Asking L. Methods of setting and measuring flow rates in pharmaceutical impactor experiments. Pharm Forum. 2003;29(3):879–84.
Roberts DL, Romay FJ. Relationship of stage mensuration data to the performance of new and used cascade impactors. J Aerosol Med. 2005;18(4):396–413.
Chambers F, Ali A, Mitchell J, Shelton C, Nichols S. Cascade impactor (CI) mensuration: an assessment of the accuracy and precision of commercially available optical measurement systems. AAPS PharmSciTech. 2010;11(1):472–84.
Svensson M, Pettersson G, Asking L. Stage mensuration of the Andersen impactor—pitfalls and recommendations. In: Drug delivery to the lungs—13. London: The Aerosol Society; 2002. p. 188–91.
Marple VA, Olson BA, Miller NC. A low-loss cascade impactor with stage collection cups: calibration and pharmaceutical inhaler applications. Aerosol Sci Technol. 1995;22(1):124–34.
Sarker DK. Quality control, analytical validation and pharmacopoeial testing. Business Briefing—Future Drug Discovery. 2004:38–41. http://www.touchbriefings.com/2A6AD410-D886-4189-8A78-6C7B2AD54803/FinalDownload/DownloadId-20339FC03D11ADFFAA11CB63631C51CD/2A6AD410-D886-4189-8A78-6C7B2AD54803/pdf/890/PT04_sarker.pdf. Accessed 4 Oct 2012.
Roberts DL, Lavarreda C. Controlling flow resistance as a means of reducing inter-impactor variability. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory drug delivery. River Grove: Davis Healthcare; 2008. p. 661–4.
Milhomme K, Dunbar C, Lavarreda C, Roberts D, Romay F. Measuring changes in the effective jet diameter of cascade impactor stages with the flow resistance monitor. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory drug delivery. River Grove: Davis Healthcare; 2006. p. 405–7.
Roberts DL, Lavarreda C, Milhomme K, Shelton C. Managing impactor quality with measurements of flow resistance and effective diameter. Drug delivery to the lungs—17. Edinburgh: The Aerosol Society; 2006. p. 243–6.
Shelton C, Roberts DL. Application of flow resistance measurements to cascade impactor quality control. Drug delivery to the lungs—16. Edinburgh: The Aerosol Society; 2005. p. 221–4.
Lavarreda C, Roberts D, Romay F. Cascade impactor qualification: evaluating the correlation between the area-mean jet diameter and the flow resistance of cascade impactor stages. Proc. 7th Int. Aerosol Conf. St. Paul, MN, USA, 2006:840.
Acknowledgments
The authors wish to acknowledge the support received through members of the Impactor Sub-Team of the European Pharmaceutical Aerosol Group (EPAG) during discussions related to the topics covered by this article. They also wish to acknowledge Copley Scientific, and Westech Instrument Services, for the supply of data and other information from time to time that helped provide the comprehensive coverage of CI characteristics reported in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nichols, S.C., Mitchell, J.P., Shelton, C.M. et al. Good Cascade Impactor Practice (GCIP) and Considerations for “In-Use” Specifications. AAPS PharmSciTech 14, 375–390 (2013). https://doi.org/10.1208/s12249-012-9905-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9905-1