Abstract
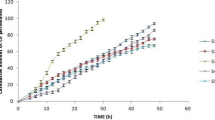
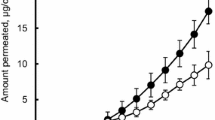
A novel drug-in-adhesive matrix was designed and prepared. A thermoplastic elastomer, styrene–isoprene–styrene (SIS) block copolymer, in combination with tackifying resin and plasticizer, was employed to compose the matrix. Capsaicin was selected as the model drug. The drug percutaneous absorption, adhesion properties, and skin irritation were investigated. The results suggested that the diffusion through SIS matrix was the rate-limiting step of capsaicin percutaneous absorption. [SI] content in SIS and SIS proportions put important effects on drug penetration and adhesion properties. The chemical enhancers had strong interactions with the matrix and gave small effect on enhancement of drug skin permeation. The in vivo absorption of samples showed low drug plasma peaks and a steady and constant plasma level for a long period. These results suggested that the possible side effects of drug were attenuated, and the pharmacological effects were enhanced with an extended therapeutic period after application of SIS matrix. The significant differences in pharmacokinetic parameters produced by different formulations demonstrated the influences of SIS copolymer on drug penetrability. Furthermore, the result of skin toxicity test showed that no skin irritation occurred in guinea pig skin after transdermal administration of formulations.









Similar content being viewed by others
REFERENCES
Webster I. Recent developments in pressure-sensitive adhesives for medical applications. Int J Adhes Adhes. 1997;17:69–73. doi:10.1016/S0143-7496(96)00024-3.
Galán C, Sierra CA, Gómez Fatou JM, Delgado JA. A hot-melt pressure-sensitive adhesive based on styrene–butadiene–styrene rubber: the effect of adhesive composition on the properties. J Appl Polym Sci. 1996;62:1263–75. doi:10.1002/(SICI)1097-4628.
Nazhat SN, Parker S, Patel MP. Isoprene–styrene copolymer elastomer and tetrahydrofurfuryl methacrylate mixtures for soft prosthetic applications. Biomaterials. 2001;22:2411–6. doi:10.1016/S0142-9612(00)00428-2.
Kim DJ, Kim HJ, Yoon G. Effect of substrate and tackifier on peeling strength of SIS (styrene–isoprene–styrene)-based HMPSAs. Int J Adhes Adhes. 2005;25:288–95. doi:10.1016/j.ijadhadn.2004.10.001.
Laurer JH, Khan SA, Spontak RJ. Morphology and rheology of SIS and SEPS triblock copolymers in the presence of a midblock-selective solvent. Langmuir. 1999;15:7947–55. doi:10.1021/la981441n.
Gibert FX, Marin G, Derail C, Allal A, Lechat A. Rheological properties of hot melt pressure-sensitive adhesives based on styrene–isoprene copolymers. PartI: a rheological model for [SIS-SI] formulations. J Adhesion. 2003;79:825–52. doi:10.1080/00218460309552.
Lee WL, Kim HD, Kim EY. Morphological reorientation by extensional flow deformation of a triblock copolymer styrene–isoprene–styrene. Curr Appl Phys. 2006;6:718–22. doi:10.1016/j.cap.2005.04.026.
Efrain RP, Michael CW. Thermodynamics of plasticized triblock copolymers. Part II: model verification by light transmittance and rheology. Polym Eng Sci. 1977;17:573–81. doi:10.1002/pen.760170814.
Zhang JY, Park YJ, Kim HY. A new approach on the thermal stability of SDS copolymer for HMPSA, Part I: oxidation kinetics for the whole process. Polym Degrad Stab. 2008;93:1008–23. doi:10.1016/j.polymdegradstab.2007.12.016.
Myoung Y, Yamazaki T, Choi HK. Permeation of ciclopirox across porcine hoof membrane: effect of pressure sensitive adhesives and vehicles. Euro J Pharm Sci. 2003;20:319–25. doi:10.1016/j.ejps.2003.07.001.
Wang CX, Han W, Tang XZ, Zhang H. Evaluation of drug release profile from patches based on styrene–isoprene–styrene block copolymer: the effect of block structure and plasticizer. AAPS PharmSciTech. 2012;13:556–67. doi:10.1208/s12249-012-9778-3.
Schmook FP, Meingassner JG, Billich A. Comparison of human skin or epidermis model with human and animal skin in in-vitro percutaneous absorption. Int J Pharm. 2001;215:51–6. doi:10.1016/S0378-5173(00)00665-7.
Fusco B, Giacovazzo M. Peppers and pain: the promise of capsaicin. Drugs (Basel). 1997;53:909–14.
Thomas NS, Panchagnula P. Transdermal delivery of zidovudine: effect of vehicles on permeation across rat skin and their mechanism of action. Euro J Pharm Sci. 2003;18:71–9. doi:10.1016/S0928-0987(02)00242-7.
Fang JY, Wu PC, Huang YB, Tsai YH. In vitro permeation study of capsaicin and its synthetic derivatives from ointment bases using various skin types. Int J Pharm. 1995;126:119–28. doi:10.1016/0378-5173(95)04105-2.
Fang JY, Wu PC, Huang YB, Tsai YH. In vivo percutaneous absorption of capsaicin, nonivamide and sodium nonivamide acetate from ointment bases pharmacokinetic analysis in rabbits. Int J Pharm. 1996;128:169–77. doi:10.1016/0378-5174(95)04274-1.
Zhang Y, Huo MR, Zhou JP, Xie SF. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Meth Prog Bio. 2010;99:306–14. doi:10.1016/j.cmpb.2010.01.007.
Frosch PJ, Schulze DA, Hoffmann M, Axthelm I, Kurte AU. Efficacy of skin barrier creams (I) the repetitive irritation test (RIT) in guinea pig. Contact Dermatitis. 1993;28:94–100.
Jibry N, Murdan S. In vivo investigation, in mice and in man, into the irritation potential of novel amphiphilogels being studied as transdermal drug carriers. Eur J Pharm Biopharm. 2004;58:107–19. doi:10.1016/j.ejpb.2004.02.013.
Zhang JH, Liu ZP, Du H, Zeng Y, Deng LD, Xing JF, et al. A novel hydrophilic adhesive matrix with self-enhancement for drug percutaneous permeation through rat skin. Pharm Res. 2009;26:1398–406. doi:10.1007/s11095-009-9850-1.
Sun YH, Fang L, Zhu M, Li W, Meng P, Li L, et al. A drug-in-adhesive transdermal patch for S-amlodipine free base: in vitro and in vivo characterization. Int J Pharm. 2009;382:165–71. doi:10.1016/j.ijpharm.2009.08.031.
Magnusson MM, Koskinen LOD. In vitro percutaneous penetration of topically applied capsaicin in relation to in vivo sensation responses. Int J Pharm. 2000;195:55–62. doi:10.1016/S0378-5173(99)00337-3.
Fang JY, Wu PC, Huang YB, Tsai YH. In vivo percutaneous absorption of capsaicin, nonivamide and sodium nonivamide acetate from ointment bases: skin erythema test and non-invasive surface recovery technique in humans. Int J Pharm. 1996;131:143–51. doi:10.1016/0378-5173(95)00178-6.
Ho HO, Huang FC, Sokoloski TD, Sheu MT. The influence of cosolvents on the in-vitro percutaneous penetration of diclofenac sodium from a gel system. J Pharm Pharmacol. 1994;46:636–42. doi:10.1111/j.2042-7158,1994.tb03873.x.
Guy RH, Hadgraft J. Rate control in transdermal delivery. Int J Pharm. 1992;82:R1–6. doi:10.1016/0378-5173(92)90183-3.
Ah YC, Choi JK, Choi YK, Ki HM, Bae JH. A novel transdermal patch incorporating meloxicam: in vitro and in vivo characterization. Int J Pharm. 2010;385:12–9. doi:10.1016/j.ijpharm.2009.10.013.
Ren CS, Fang L, Ling L, Wang Q, Liu SH, Zhao LG, et al. Design and in vivo evaluation of an indapamide transdermal patch. Int J Pharm. 2009;370:129–35. doi:10.1016/j.ijpharm.2008.12.2004.
Poh BT, Kwo HK. Shear strength of SMR-based pressure-sensitive adhesives. Polym-Plast Techol. 2007;46:1021–4. doi:10.1080/03602550701521957.
Yat HK, Dodou K. Rheological studies on pressure-sensitive silicone adhesives and drug-in-adhesive layers as a means to characterize adhesive performance. Int J Pharm. 2007;333:24–33. doi:10.1016/j.ijpharm.2006.09.043.
Poh BT, Heng SS. Effect of blend ratio on adhesion properties of pressure-sensitive adhesives prepared from SBR/SMR L blends. Polym-Plast Technol. 2008;47:325–9. doi:10.1080/036202550701870081.
Wokovich AM, Prodduturi S, Doub WH, Hussain AS, Buhse LF. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur J Pharm Biopharm. 2006;64:1–8. doi:10.1016/j.ejpb.2006.03.009.
Cheong HA, Choi HK. Effect of ethanolamine salts and enhancers on the percutaneous absorption of piroxicam from a pressure sensitive adhesive matrix. Euro J Pharm Sci. 2003;18:149–53. doi:10.1016/S0928-0987(02)00254-3.
Qvist MH, Ulla H, Kreiligaard B, Madesn F, Frokajer S. Release of chemical permeation enhancers from drug-in-adhesive transdermal patches. Int J Pharm. 2002;231:253–63. doi:S0378-5173(01)00893-6.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliver Rev. 2004;56:603–18. doi:10.1016/j.addr.2003.10.025.
Fang JY, Fang CL, Hong CT, Chen HY, Lin TY, Wei HM. Capsaicin and nonivamide as novel skin permeation enhancers for indomethacin. Euro J Pharm Sci. 2001;12:195–203. doi:10.1016/S0928-0987(00)00118-4.
Zhao LG, Li Y, Fang L, He ZG, Liu XT, Wang L, et al. Transdermal delivery of tolterodine by O-acylmenthol: in vitro/in vivo correlation. Int J Pharm. 2009;374:73–81. doi:10.1016/j.ijpharm.2009.03.005.
Zhao JH, Fu JH, Wang SM, Su CH. A novel transdermal patch incorporating isosorbide dinitrate with bisoprolol: in vitro and in vivo characterization. Int J Pharm. 2007;337:88–101. doi:10.1016/j.ijpharm.2006.12.030.
Wang YY, Hong TC, Chiu WT, Fang JY. In vitro and in vivo evaluations of topically applied capsaicin and nonivamide from hydrogels. Int J Pharm. 2001;224:89–104. doi:S0378-5173(01)00755-4.
ACKNOWLEDGMENTS
This research was supported by the National Science and Technology Key Program for the 11th Five-Year Plan of China (2008BAI53B075).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Liu, R., Tang, X. et al. A Drug-in-Adhesive Matrix Based on Thermoplastic Elastomer: Evaluation of Percutaneous Absorption, Adhesion, and Skin Irritation. AAPS PharmSciTech 13, 1179–1189 (2012). https://doi.org/10.1208/s12249-012-9849-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9849-5