Abstract
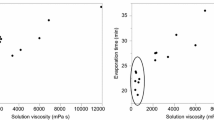
This study investigated the effects of polymer dispersion and hydration conditions on hypromellose (HPMC) film properties, such as strength, oxygen permeability, water vapor transmission, clarity, and haze. The focus of the study was to build a better understanding of the impact that changes to HPMC dispersion and hydration conditions have on performance properties of the resulting films. This understanding could potentially lead to more flexible formulation guidelines for formulators. Films of HPMC 2906 (USP) were produced from aqueous solutions prepared using various formulation conditions. Results showed that tensile properties and oxygen permeability were not significantly affected by the variables used. The differences observed in water vapor transmission are unlikely to affect practical application of the material. However, the differences observed in clarity and haze at 50°C hydration temperature could affect the appearance of a capsule or coated tablet. Several methods were used to determine whether loss of optical properties was due to surface phenomena or bulk defects within a film. Results indicated that the cloudy appearance was primarily due to surface roughness. Based on this information, there is some flexibility in formulation conditions; however, hydration temperatures greater than 25°C are not recommended.











Similar content being viewed by others
Notes
™Trademark of The Dow Chemical Company (“Dow”) or an affiliated company of Dow
References
The Dow Chemical Company. Methocel Cellulose Ethers Technical Handbook. Form No. 192-01062-0902 AMS, Sept. 2002.
Bae HJ, Cha DS, Whiteside WS, Park HJ. Film and pharmaceutical hard capsule formation properties of mungbean, waterchestnut, and sweet potato starches. Food Chem. 2008;106(1):96–105.
Al-Tabakha MM. HPMC capsules: current status and future prospects. J Pharm Pharm Sci. 2010;13(3):428–42.
Felton LA, Timmins GS. A nondestructive technique to determine the rate of oxygen permeation into solid dosage forms. Pharm Dev Technol. 2006;11(1):141–7.
Missaghi S, Fassihi R. Evaluation and comparison of physicomechanical characteristics of gelatin and hypromellose capsules. Drug Devel Ind Pharm. 2006;32(7):829–38.
Debeaufort F, Voilley A, Meares P. Water vapor permeability and diffusivity through methylcellulose edible films. J Membrane Sci. 1994;91(6):125–33.
Laksmana F, Hartman Kok P, Frijlink H, Vromans H, Van Der Voort Maarschalk K. Gas permeation related to the moisture sorption in films of glassy hydrophilic polymers. J Appl Polym Sci. 2010;116(6):3310–7.
Johnson M, Wilkes G, Sukhadia A, Rohlfing D. Optical properties of blown and cast polyethylene films: surface versus bulk structural considerations. J Appl Polym Sci. 2000;77(13):2845–64.
Larena A, Pinto G. The effect of surface roughness and crystallinity on the light scattering of polyethylene tubular blown films. Polym Eng Sci. 1993;33(12):742–7.
Stehling FC, Speed CS, Westerman L. Causes of haze of low-density polyethylene blown films. Macromolecules. 1981;14(3):698–708.
Ashizawa H, Spruiell JE, White JL. An investigation of optical clarity and crystalline orientation in polyethylene tubular film. Polym Eng Sci. 1984;24(13):1035–42.
Seitavoupio P, Heinamaki J, Rantanen J, Yliruusi J. Monitoring tablet surface roughness during the film coating process. Pharm Sci Technol. 2006;7(2):E1–6.
Sarkar N. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. J Appl Polym Sci. 1979;24(4):1073–87.
Acknowledgments
We would like to thank Bob Gunther and Sheri Clark for assistance with measuring clarity and haze properties of the films, Ellen Keene for conducting the microscopy oil test, and Meagan Blake for profilometry analysis of the films.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Curtis-Fisk, J., Sheskey, P., Balwinski, K. et al. Effect of Formulation Conditions on Hypromellose Performance Properties in Films Used for Capsules and Tablet Coatings. AAPS PharmSciTech 13, 1170–1178 (2012). https://doi.org/10.1208/s12249-012-9841-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9841-0