Abstract
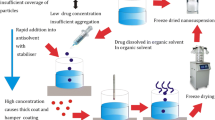
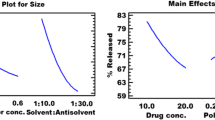
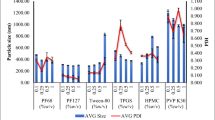
The present study was performed to investigate potential of Eudragit RLPO-based nanosuspension of glimepiride (Biopharmaceutical Classification System class II drug), for the improvement of its solubility and overall therapeutic efficacy, suitable for peroral administration. Nanoprecipitation method being simple and less sophisticated was optimized for the preparation of nanosuspension. Physicochemical characteristics of nanosuspension in terms of size, zeta potential, polydispersity index, entrapment efficiency (% EE) and in vitro drug release were found within their acceptable ranges. The size of the nanoparticles was most strongly affected by agitation time while % EE was more influenced by the drug/polymer ratio. Differential scanning calorimetry and X-ray diffraction studies provided evidence that enhancement in solubility of drug resulted due to change in crystallinity of drug within the formulation. Stability study revealed that nanosuspension was more stable at refrigerated condition with no significant changes in particle size distribution, % EE, and release characteristics for 3 months. In vivo studies were performed on nicotinamide–streptozotocin-induced diabetic rat models for pharmacokinetic and antihyperglycaemic activity. Nanosuspension increased maximum plasma concentration, area under the curve, and mean residence time values significantly as compared to aqueous suspension. Oral glucose tolerance test and antihyperglycaemic studies demonstrated plasma glucose levels were efficiently controlled in case of nanosuspension than glimepiride suspension. Briefly, sustained and prolonged activity of nanosuspensions could reduce dose frequency, decrease drug side effects, and improve patient compliance. Therefore, glimepiride nanosuspensions can be expected to gain considerable attention in the treatment of type 2 diabetes mellitus due to its improved therapeutic activity.













Similar content being viewed by others
REFERENCES
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Formulation and biological evaluation of glimepiride–cyclodextrin–polymer systems. Int J Pharm. 2006;309(1–2):129–38.
Frick A, Moller H, Wirbitzki E. Biopharmaceutical characterization of oral immediate release drug products. In vitro/in vivo comparison of phenoxymethylpenicillin potassium, glimepiride and levofloxacin. Eur J Pharm Biopharm. 1998;46(3):305–11.
Ilic I, Dreu R, Burjak M, Homar M, Kerc J, Srcic S. Microparticle size control and glimepiride microencapsulation using spray congealing technology. Int J Pharm. 2009;381(2):176–83.
Babu RJ, Pandit JK. Effect of aging on the dissolution stability of glibenclamide/beta-cyclodextrin complex. Drug Dev Ind Pharm. 1999;25(11):1215–9.
Reven S, Grdadolnik J, Kristl J, Zagar E. Hyperbranched poly(esteramides) as solubility enhancers for poorly water-soluble drug glimepiride. Int J Pharm. 2010;396(1–2):119–26.
Junghanns JUAH, Muller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–309.
Kesisoglou F, Panmai S, Wu Y. Nanosizing—oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59(7):631–44.
Jacobs C, Muller RH. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002;19(2):189–94.
Kipp JE. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm. 2004;284(1–2):109–22.
Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol. 2008;36(1):43–8.
Verma S, Gokhale R, Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380(1–2):216–22.
Muller RH, Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002;237(1–2):151–61.
Reichal CR, Lakshmi JB, Ravi TK. Studies on formulation and in vitro evaluation of glimepiride floating tablets. J Chem Pharm Res. 2011;3(3):159–64.
Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–4.
Guterres SS, Fessi H, Barratt G, Devissaguet JP, Puisieux F. Poly(dl-lactide) nanocapsules containing diclofenac: I. Formulation and stability study. Int J Pharm. 1995;113(1):57–63.
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58(15):1688–713.
Omari DM, Sallam A, Abd-Elbary A, El-Samaligy M. Lactic acid-induced modifications in films of Eudragit RL and RS aqueous dispersions. Int J Pharm. 2004;274(1–2):85–96.
Amlathe S, Gupta VK. Spectrophotometric determination of acetone using vanillin. Analyst. 1990;115(10):1385–7.
Chorny M, Fishbein I, Danenberg HD, Golomb G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Control Release. 2002;83(3):389–400.
Mishra B, Arya N, Tiwari S. Investigation of formulation variables affecting the properties of lamotrigine nanosuspension using fractional factorial design. Daru. 2010;18(1):1–8.
Rabbaa-Khabbaz L, Daoud RA, Karam-Sarkis D, Atallah C, Zoghbi A. A simple and sensitive method for determination of glimepiride in human serum by HPLC. J Liq Chromatogr Relat Technol. 2005;28(20):3255–63.
Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47(2):224–9.
Shirwaikar A, Rajendran K, Kumar CD, Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin-nicotinamide type 2 diabetic rats. J Ethnopharmacol. 2004;91(1):171–5.
Lott JA, Turner K. Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin Chem. 1975;21(12):1754–60.
Kuppusamy A, Muthusamy U, Thirumalaisamy SA, Varadharajan S, Ramasamy K, Ramanathan S. In vitro (α-glucosidase and α-amylase inhibition) and in vivo antidiabetic property of phytic Acid (IP6) in streptozotocin–nicotinamide-induced type 2 diabetes mellitus (NIDDM) in rats. J Complement Integr Med. 2011;8(1):doi:10.2202/1553-3840.1483.
Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G. Preparation and characterization of Eudragit retard nanosuspensions for the ocular delivery of cloricromene. AAPS PharmSciTech. 2006;7(1):E192–8. doi:10.1208/pt070127.
Dillen K, Vandervoort J, Mooter GV, Ludwig A. Evaluation of ciprofloxacin-loaded Eudragit® RS100 or RL100/PLGA nanoparticles. Int J Pharm. 2006;314(1):72–82.
Murakami H, Kobayashi M, Takeuchi H, Kawashima Y. Preparation of poly(dl-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int J Pharm. 1999;187(2):143–52.
Ubrich N, Schmidt C, Bodmeier R, Hoffman M, Maincent P. Oral evaluation in rabbits of cyclosporin-loaded Eudragit RS or RL nanoparticles. Int J Pharm. 2005;288(1):169–75.
Bayems V, Gurny R. Chemical and physical parameters of tears relevant for the design of ocular drug delivery formulations. Pharm Acta Helv. 1997;72(4):191–202.
Das S, Suresh PK, Desmukh R. Design of Eudragit RL 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomedicine. 2010;6(2):318–23.
Thioune O, Fessi H, Devissaguet JP, Puisieux F. Preparation of pseudolatex by nanoprecipitation: influence of the solvent nature on intrinsic viscosity and interaction constant. Int J Pharm. 1997;146(2):233–8.
Passerini N, Albertini B, Gonzalez-Rodriguez ML, Cavallari C, Rodriguez L. Preparation and characterisation of ibuprofen-poloxamer 188 granules obtained by melt granulation. Eur J Pharm Sci. 2002;15(1):71–8.
Liu D, Fei X, Wang S, Jiang T, Su D. Increasing solubility and dissolution rate of drugs via eutectic mixtures: itraconazole-poloxamer188 system. Asian J Pharm Sci. 2006;1(3–4):213–21.
Glaessl B, Siepmann F, Tucker I, Siepmann J, Rades T. Characterisation of quaternary polymethacrylate films containing tartaric acid, metoprolol free base or metoprolol tartrate. Eur J Pharm Biopharm. 2009;73(3):366–72.
Dandagi P, Kerur S, Mastiholimath V, Gadad A, Kulkarni A. Polymeric ocular nanosuspension for controlled release of acyclovir: in vitro release and ocular distribution. IJPR. 2009;8(2):79–86.
Xie J, Wang CH. Self-assembled biodegradable nanoparticles developed by direct dialysis for the delivery of paclitaxel. Pharm Res. 2005;22(12):2079–90.
Kim YI, Fluckiger L, Hoffman M, Lartaud-Idjouadiene I, Atkinson J, Maincent P. The antihypertensive effect of orally administered nifedipine-loaded nanoparticles in spontaneously hypertensive rats. Br J Pharmacol. 1997;120(3):399–404.
Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatment for diabetes: studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33(8):462–4.
Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, et al. Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med. 2008;44(4):584–93.
ACKNOWLEDGMENTS
The first author acknowledges financial assistance from University Grants Commission (UGC), New Delhi, for carrying out this research work. All authors would like to acknowledge Department of Applied Physics and Department of Chemistry, Banaras Hindu University, India for providing facility of XRD and DSC. We are also thankful to Alkem Laboratories, Lupin Pharma Labs and Ranbaxy laboratories for providing gift samples of drug and polymers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, S.K., Mishra, S. & Mishra, B. Eudragit-Based Nanosuspension of Poorly Water-Soluble Drug: Formulation and In Vitro–In Vivo Evaluation. AAPS PharmSciTech 13, 1031–1044 (2012). https://doi.org/10.1208/s12249-012-9833-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9833-0