Abstract
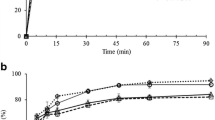
The dissolution test for oral dosage forms has recently widened to a variety of special dosage forms such as suspensions. For class II drugs, such as nimesulide (NMS), this study is very important because formulation problems may compromise drug bioavailability. In the present work, tests with four brands of commercially available NMS (RA, TS, TB, and TC) have been performed in order to study their dissolution at different conditions. The suspensions have been characterized relatively to particle size, pH, and density besides NMS assay and the amount of drug in solution in the suspension vehicles. The dissolution study was conducted using the following media: simulated intestinal fluid, pH 6.8, containing polysorbate 80 (P80) or sodium lauryl sulfate (SLS); phosphate buffer, pH 7.4, with P80 and aqueous solution of SLS. Concerning the quantitative analysis, the UV–VIS spectrophotometry could have been used in substitution to high-performance liquid chromatography since the methodology had been adequately validated. The influence of the drug particle size distribution was significant on the dissolution profiles of NMS formulations, confirming to be a factor that should be strictly controlled in the development of oral suspensions.





Similar content being viewed by others
References
Siewert M, Dressman J, Brown CK, Shah VP. FIP/AAPS guidelines to dissolution in vitro release testing of novel special dosage forms. AAPS PharmSciTech. 2003;7:1–10.
Jamzad S, Fassihi R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—a technical note. AAPS PharmSciTech. 2006;33:E1–6.
Fallavena PRB, Schapoval EES. pK a determination of nimesulide in methanol–water mixtures by potentiometric titrations. Int J Pharm. 1997;158:109–12.
Singh S, Sharda N, Mahajan L. Spectrophotometric determination of pK a of nimesulide. Int J Pharm. 1999;176:261–4.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theorical basic for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution in vivo bioavailability. Pharm Res. 1995;12:413–20.
Brown CK, Chokshi HP, Nickerson B, Reed RA, Rohrs BR, Shah PA. Acceptable analytical practices for dissolution testing of poorly soluble compounds. Pharm Technol. 2004;12:56–65.
He Z, Zhong D, Chen X, Liu X, Tang X, Zhao L. Development of a dissolution medium for nimodipine tablets based on bioavailability evaluation. Eur J Pharm Sci. 2004;21:487–91.
International Conference on Harmonisation (ICH). Validation of analytical procedures: text and methodology Q2(R1). Geneva: ICH Secretariat; 2005.
The United States Pharmacopeia, Reagents, USP Convention, Rockville, USA; 2006. p. 3167–8.
Jenke DR. Chromatographic method validation: a review of current practices and procedures. II. Guidelines for primary parameters. J Liq Chromatogr Relat Technol. 1996;19:737–57.
Fortunato D. Dissolution method development for immediate release solid oral dosage forms. Dissolution Technologies; 2005. p. 12–4.
Lindenberg M, Wiegand C, Dressman JB. Comparison of the adsorption of several drugs to typical filter materials. Dissolution Technologies; 2005. p. 22–5.
Oliveira MRS. MSc thesis in Pharmaceutical Sciences, Faculty of Pharmacy, Federal University of Rio de Janeiro, Brazil; 2003. p. 101.
Silva RL, Volpato NM. Meios para dissolução de comprimidos de nimesulida: ação dos tensoativos. Braz J Pharm Sci. 2002;38:163–72.
CDER/FDA. Guidance for industry. Dissolution testing of immediate release solid oral dosage forms; 1997.
Acknowledgments
The authors would like to thank CAPES, Brazil for fellowship conferred to L.B. Fonseca, to LabCQ-UFRJ for financial support, and to Schering-Plough for supplying the reference substance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Fonseca, L.B., Labastie, M., de Sousa, V.P. et al. Development and Validation of a Discriminative Dissolution Test for Nimesulide Suspensions. AAPS PharmSciTech 10, 1145–1152 (2009). https://doi.org/10.1208/s12249-009-9320-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9320-4