Abstract
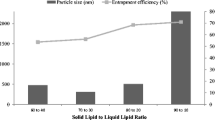
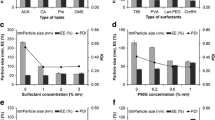
The objective of the present investigation was to formulate solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for improving the dermal delivery of a local anesthetic agent lidocaine (LID). SLN and NLC were characterized for particle size distribution, polydispersity index, entrapment efficiency, X-ray powder diffraction pattern (XRD), thermal behavior by differential scanning colorimeter (DSC) and surface morphology by transmission electron microscopy (TEM). LID-loaded SLN and NLC were formulated into hydrogels for topical application. The in vitro permeation profiles of LID SLN gel, LID NLC gel, and a marketed LID formulation (Xylocaine® gel) were evaluated by using guinea pig skin. The in vivo efficacy of LID SLN gel, LID NLC gel, and a marketed LID formulation (Xylocaine® gel) gel was evaluated on guinea pig using pinprick test. LID SLN showed a particle size of 78.1 nm with a polydispersity index of 0.556, whereas LID NLC showed a particle size of 72.8 nm with a polydispersity index of 0.463. The entrapment efficiency of LID in both SLN and NLC was 97% and 95.9%, respectively. The TEM studies revealed the almost spherical nature of LID SLN and NLC formulations. The XRD and DSC studies of LID SLN suggested amorphization of drug in the carrier system. The SLN formulation was stable with respect to particle size, polydispersity, and entrapment efficiency for 6 months at 40°C/75% relative humidity (RH). Negligible leakage was observed for the NLC formulation when stored for 1 month at 40°C/75% RH. In vitro permeation studies indicated that LID SLN gel and LID NLC gel significantly sustained the LID release compared to that of Xylocaine® gel. The in vivo efficacy results supported the results of the in vitro permeation studies wherein the LID SLN gel and LID NLC gel resulted in fivefold and sixfold increase in duration of anesthesia, respectively, compared to that of Xylocaine® gel.






Similar content being viewed by others
References
Wei H, Chen Y, Xu L, Zheng J. Percutaneous penetration kinetics of lidocaine and prilocaine in two local anesthetic formulations assessed by in vivo microdialysis in pigs. Biol Pharm Bull. 2007;30(4):830–4.
Rolland A. Particulate carriers in dermal and transdermal drug delivery: myth or reality? In: Rolland A, editor. Pharmaceutical particulate carriers—therapeutic applications. New York: Marcel Dekker; 1993. p. 367–417.
Barratt G. Colloidal drug carriers: achievements and perspectives. Cell Mol Life Sci. 2003;60:21–37.
Schmid MH, Korting HC. Therapeutic process with topical liposome drugs for skin disease. Adv Drug Deliv Rev. 1996;18:335–42.
Nagarsenker MS, Joshi AA. Preparation, characterization and evaluation of liposomal dispersions of lidocaine. Drug Dev Ind Pharm. 1997;23(12):1159–65.
Kreilgaard M, Pedersen EJ, Jaroszewski JW. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J Control Release. 2000;69:421–33.
Sintov AC, Brandys-Sitton R. Facilitated skin penetration of lidocaine: combination of a short-term iontophoresis and microemulsion formulation. Int J Pharm. 2006;316:58–67.
Trumbore MW. A comparison of the rate and extent of lidocaine release from a novel topical anesthetic foam vs. a currently marketed lidocaine 4% cream. AAD Meeting February 2008.
Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–78.
Mehnert W, Mader K. Solid lipid nanoparticles production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96.
Wissing SA, Muller RH. Cosmetic applications for solid lipid nanoparticles (SLN). Int J Pharm. 2003;254:65–8.
Maia CS, Mehnert W, Schaller M, Korting HC, Gysler A, Haberland A, et al. Drug targeting by solid lipid nanoparticles for dermal use. J Drug Target. 2002;10:489–95.
Chen H, Chang X, Du D, Liu W, Liu J, Weng T, et al. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Control Release. 2006;110:296–306.
Lopes LB, Ferreira DA, Paula D, Garcia MJ, Thomazini JA, Fantini MA, et al. Reverse hexagonal phase nanodispersion of monoolein and oleic acid for topical delivery of peptides: in vitro and in vivo skin penetration of cyclosporin A. Pharm Res. 2006;23:1332–42.
Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328:191–5.
Munster U, Nakamura C, Haberland A, Jores K, Mehnert W, Rummel S, et al. RU 58841-myristate—prodrug development for topical treatment of acne and androgenetic alopecia. Pharmazie. 2005;60:8–12.
Iannuccelli V, Sala N, Tursilli R, Coppi G, Scalia S. Influence of liposphere preparation on butyl-methoxydibenzoylmethane photostability. Eur J Pharm Biopharm. 2006;63:140–5.
Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007;345:163–71.
Muller RH, Mader K, Golha S. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–55.
Foldvari M, Gesztes A, et al. Topical liposomal local anesthetics: design, optimization and evaluation of formulations. Drug Dev Ind Pharm. 1993;19(19):2499–517.
Muller RH, Heinemann S. Fat emulsions for parenteral nutrition. I. Evaluation of microscopic and laser light scattering methods for the determination of the physical stability. Clin Nutr. 1992;11:223–36.
Freitas C, Muller RH. Correlation between long-term stability of solid lipid nanoparticles (SLN™) and crystallinity of the lipid phase. Eur J Pharm Biopharm. 1999;47:125–32.
Jenning V, Gohla S. Solid lipid nanoparticles (SLN) based on the binary mixtures of liquid and solid lipids: a 1H-NMR study. Int J Pharm. 2000;205:15–21.
Jenning V, Schäfer-Korting M, Gohla S. Vitamin A loaded solid lipid nanoparticles for topical use: drug release properties. J Control Release. 2000;66:115–26.
Freitas C, Muller RH. Stability determination of solid lipid nanoparticles (SLN™) in aqueous dispersion after addition of electrolyte. J Microencapsul. 1999;16:59–71.
Acknowledgements
The authors would like to acknowledge Exim Pharm International, Heer Pharma, Colorcon Asia, S. Zhaveri and Sons, and Noveon for providing us the gift samples of drug and excipients. Also, we would like to acknowledge the kind help of Mr. Nilesh Kulkarni and Mr. Chopre of Tata Institute of Fundamental Research (TIFR), Mumbai, India in conducting the XRD studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pathak, P., Nagarsenker, M. Formulation and Evaluation of Lidocaine Lipid Nanosystems for Dermal Delivery. AAPS PharmSciTech 10, 985–992 (2009). https://doi.org/10.1208/s12249-009-9287-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9287-1