Abstract
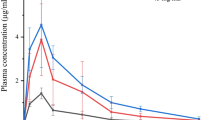
The purpose of this research was to develop an emulsion formulation of indomethacin (IND) suitable for nasal delivery. IND was incorporated into the oil phases of oil in water (O/W) and water in oil (W/O) emulsions. For this purpose, different emulsifying agents (Tween 80, Span 80 and Brij 58) were used in two emulsion formulations. When the effects of several synthetic membranes (nylon, cellulose, cellulose nitrate) were compared with the sheep nasal mucosa, the cellulose membrane and sheep nasal mucosa showed similar permeation properties for O/W emulsion (P > 0.05). To examine the absorption characteristics of IND, the anti-inflammatory properties of intravenous solution of IND, intranasal O/W emulsions of IND (with or without enhancers) and intranasal solution of IND (IND-Sol) were investigated in rats with carrageenan-induced paw edema. When citric acid was added to the nasal emulsion, the anti-inflammatory activity was similar to that of intravenous solution (P > 0.05). Finally, it was concluded that, intranasal administration of IND emulsion with citric acid may be considered as an alternative to intravenous and per oral administrations of IND to overcome their adverse effects.




Similar content being viewed by others
References
Y. W. Chien, K. S. E. Su, and S. F. Chang. Nasal Systemic Drug Delivery, Marcel Dekker, New York, 1989, pp. 1–310.
M. Tafaghodi, S. A. S. Tabassi, M. R. Jaafari, S. R. Zakavi, and M. Momen-nejad. Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. Int. J. Pharm 280:125–135 (2004).
M. Hinchcliffe, and L. Illum. Intranasal insulin delivery and therapy. Adv. Drug Deliv. Rew 35:199–234 (1999).
K. Aikawa, K. Matsumoto, N. Mitsutake, H. Uda, S. Tanaka, H. Shimamura, Y. Aramaki, and S. Tsuchiya. Drug release from pH-response polymer to nasal delivery. STP Pharma Sci 12:69–74 (2002).
R. Mitra, I. Pezron, W. A. Chu, and A. K. Mitra. Lipid emulsions as vehicles for enhanced nasal delivery of insulin. Int. J. Pharm 205:127–134 (2000).
G. S. Tirucherai, I. Pezron, and A. K. Mitra. Novel approaches to nasal delivery of peptides and proteins. STP Pharma Sci 12:3–12 (2002).
L. Illum, A. N. Fisher, I. Jabbal-Gill, and S. S. Davis. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the nasal absorption of polypeptides. Int. J. Pharm 222:109–119 (2001).
P. Kan, Z. B. Chen, R. Y. Kung, C. J. Lee, and I. M. Chu. Study on the formulation of o/w emulsion as carriers for lipophilic drugs. Colloids Surf B: Biointerfaces 15:117–125 (1999).
T. Matsuyama, T. Morita, Y. Horikiri, H. Yamahara, and H. Yoshino. Improved nasal absorption of salmon calcitonin by powdery formulation with N-acetyl-l-cysteine as a mucolytic agent. J. Control. Release 115:183–188 (2006).
Z. L. Huang, M. Kagoshima, E. Kagawa, and H. Shimada. Absorption of indomethacin from nasal cavity in rats. Acta. Pharmacol. Sin 16:117–120 (1995).
G. Yetkin, N. Celebi, C. Ozogul, and A. T. Demiryurek. Enhancement of nasal absorption of salmon calcitonin in rabbits using absorption enhancer. STP Pharma Sci 11:187–191 (2001).
J. Y. Fang, T. L. Hwang, and Y. L. Leu. Effect of enhancers and retarders on percutaneous absorption of flurbiprofen from hydrogels. Int. J. Pharm 250:313–325 (2003).
P. Sinswat, and P. Tengamnuay. Enhancing effect of chitosan on nasal absorption of salmon calcitonin in rats: comparison with hydroxypropyl- and dimethyl-b-cyclodextrins. Int. J. Pharm 257:15–22 (2003).
C. Hascicek, N. Gonul, and N. Erk. Mucoadhesive microspheres containing gentamicin sulfate for nasal administration: preparation and in vitro characterization. Farmaco 58:11–16 (2003).
C. Tas, Y. Ozkan, A. Savaser, and T. Baykara. In vitro release studies of chlorpheniramine maleate from gels prepared by different cellulose derivatives. Farmaco 58:605–611 (2003).
S. P. Jones, N. A. Greenway, and N. A. Orr. The influence of receptor fluid on in vitro percutaneous penetration. Int. J. Pharm 53:43–46 (1989).
L. A. M. Ferreira, M. Seiller, J. L. Grossiord, J. P. Marty, and J. Wepierre. Vehicle influence on in vitro release of glucose: w/o, w/o/w and o/w systems compared. J. Control. Release 33:349–356 (1995).
S. Lang, B. R. Rutishauser, J. C. Perriard, M. C. Schmidt, and H. P. Merkle. Permeation and pathways of human calcitonin (hCT) across excised bovine nasal mucosa. Peptides 19:599–607 (1998).
T. Kissel, and U. Werner. Nasal delivery of peptides: an in vitro cell culture model for the investigation of transport and metabolism in human nasal epithelium. J. Control. Release 53:195–203 (1998).
Z. Mei, H. Chen, T. Weng, Y. Yang, and X. Yang. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur. J. Pharm. Biopharm 56:189–196 (2003).
P. Fernandez, V. André, J. Rieger, and A. Kühnle. Nano-emulsion formulation by emulsion phase inversion. Colloids Surf A: Physicochem Eng Asp 251:53–58 (2004).
V. B. Junyaprasert, P. Boonsaner, S. Leatwimonlak, and P. Boonme. Enhancement of the skin permeation of clindamycin phosphate by aerosol OT/1-butanol microemulsions. Drug Dev. Ind. Pharm 33:874–880 (2007).
S. Peltola, P. Saarinen-Savolainen, J. Kiesvaara, T. M. Suhonen, and A. Urtti. Microemulsions for topical delivery of estradiol. Int. J. Pharm 254:99–107 (2003).
M. I. Ugwoke, E. Sam, G. V. D. Mooter, N. Verbeke, and R. Kingest. Nasal mucoadhesive delivery systems of the antiparkinsonian drug, apomorfine: Influence of drug-loading on in vitro and in vivo release in rabbits. Int. J. Pharm 91:125–138 (1999).
J. P. Longenecker, A. C. Moses, J. S. Flier, R. D. Silver, M. C. Carey, and E. J. Dubovi. Effects of sodium taurodihydrofusidate on nasal absorption of insulin in sheep. J. Pharm. Sci 76:351–355 (1987).
H. Todo, H. Okamoto, K. Lida, and K. Danjo. Effect of additives on insulin absorption from intratracheally administered dry powders in rats. Int. J. Pharm 220:101–110 (2001).
H. Todo, H. Okamoto, K. Lida, and K. Danjo. Improvement of stability and absorbability of dry insulin powder for inhalation by powder-combination technique. Int J Pharm 271:41–52 (2004).
M. Hayashi, T. Sakai, Y. Hasegawa, T. Nishikawahara, H. Tomioka, A. Lida, N. Shimizu, M. Tomita, and S. Awazu. Physiological mechanism for enhancement of paracellular drug transport. J. Control. Release 62:141–148 (1999).
I. Ozguney-Sarıgullu, H. Y. Karasulu, G. Kantarcı, S. Sözer, T. Güneri, and G. Ertan. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS PharmSciTech 7:88 (2006).
Acknowledgements
This study was supported by Research Foundation of Ege University. The authors would like to thank Pınar Integrated Meat and Flour Inc. for providing the sheep nasal mucosa in performing the ex vivo studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karasulu, H.Y., Şanal, Z.E., Sözer, S. et al. Permeation Studies of Indomethacin from Different Emulsions for Nasal Delivery and Their Possible Anti-Inflammatory Effects. AAPS PharmSciTech 9, 342–348 (2008). https://doi.org/10.1208/s12249-008-9053-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-008-9053-9