Abstract
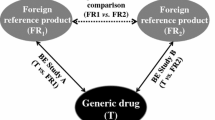
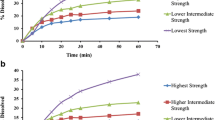
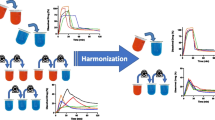
Dissolution experiments to support an active pharmaceutical ingredient (API) form change in Verubecestat immediate release tablets were performed following current regulatory guidance published by health authorities in Canada, Australia, Japan, the EU, and the USA. Verubecestat API meets the requirements of a Biopharmaceutics Classification System class 1 compound and tablets are very rapidly dissolving in aqueous dissolution media. While the in vitro data were reviewed favorably by these agencies, the divergence in regulatory requirements led to unnecessary work and highlights several issues companies operating globally face to justify product changes that have very little impact on quality. The data presented in this manuscript provide a compelling case for adjustments to the current draft ICH M9 guidance which provides recommendations for biowaiver applications. Specifically, this manuscript contains recommendations with respect to API attributes, selection of dissolution media and apparatus, and methods to assess dissolution similarity if needed, which should be considered for inclusion in a science- and risk-based global guidance document to benefit patients, regulators, and the pharmaceutical industry.



Similar content being viewed by others
Notes
At the time when the API form change was discovered, the requirements described in the 2015 Draft US FDA BCS biowaiver guidance were followed. The experiments are the same as those that are required in the current guidance.
Abbreviations
- API:
-
Active Pharmaceutical Ingredient
- BCS:
-
Biopharmaceutics Classification System
- CMA:
-
Critical Materials Attribute
- CPP:
-
Critical Process Parameter
- CQA:
-
Critical Quality Attribute
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- ICH:
-
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use
- IR:
-
Immediate Release
- MR:
-
Modified Release
- SUPAC:
-
Scale-up and Post-approval Change
- PBBM:
-
Physiologically Based Biopharmaceutics Model
- PK:
-
Pharmacokinetic
- QC:
-
Quality Control
- XRPD:
-
X-ray Powder Diffraction
- UPLC:
-
Ultra-performance Liquid Chromatography
- USP:
-
United States Pharmacopeia
- JP:
-
Japanese Pharmacopeia
- PMDA:
-
Pharmaceutics and Medical Device Agency (Japanese health agency)
- ANVISA:
-
Agência Nacional de Vigilância Sanitária (National Health Surveillance Agency Brazil)
- RDC:
-
Resolução da Diretoria Colegiada (Resolution of the Collegiate Board)
- TGA:
-
Therapeutic Goods Agency (Australian Health Agency)
- HC:
-
Health Canada
- UV:
-
Ultraviolet
- rpm:
-
Revolutions per minute
- NF:
-
National Formulary
- logP:
-
Partition Coefficient
- P eff :
-
Effective Permeability
- Vc:
-
Central Volume of Distribution
- K 12/K 21 :
-
Distribution Rate Constants
- f up :
-
Unbound Fraction in Plasma
- CL:
-
Systemic Clearance
References
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
European Medicines Agency. Reflection paper on the dissolution specification for generic solid oral immediate release products with systemic action. 2017.
National Health Surveillance Agency of Brazil (ANVISA). Guide no. 14/2018 - version 1, from February 08, 2018. Dissolution guide applicable to generic, new and similar medicines. 2018.
U.S. Department of Health and Human Services - Food and Drug Administration CDER. Dissolution testing and specification criteria for immediate-release solid oral dosage forms containing biopharmaceutics classification system class 1 and 3 drugs - guidance for industry. 2018.
U.S. Department of Health and Human Services - Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry: immediate release solid oral dosage forms: scale-up and postapproval changes: chemistry, manufacturing, and controls; in vitro dissolution testing and in vivo bioequivalence documentation. 1995.
European Comission. Guidelines on the details of the various categories of variations, on the operation of the procedures laid down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 of 24 November 2008 concerning the examination of variations to the terms of marketing authorisations for medicinal products for human use and veterinary medicinal products and on the documentation to be submitted pursuant to those procedures.: Official Journal of the European Union. 2013.
National Health Surveilance Agency of Brazil (ANVISA). Resolution RDC no 73. Provides guidelines for post-registration changes, cancellation of registration of medications with synthetic and semi-synthetic active ingredients and other measures. Dissolution guide applicable to generic, new and similar medicines. 2016.
European Medicines Agency. Guideline on the investigation of bioequivalence. 2010.
World Health Organization. WHO technical report series. No. 992 annex 7. Multisource (Generic) pharmaceutical products: guidelines on registration requirements to establish interchangeability. 2015. http://apps.who.int/medicinedocs/documents/s21898en/s21898en.pdf. Accessed 28Nov.2019.
U.S. Department of Health and Human Services - Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system - guidance for industry. FDA. 2017.
Health Canada. Guidance document: biopharmaceutics classification system based biowaiver. 2014. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/biopharmaceutics-classification-system-based-biowaiver.html. Last accessed 28Nov.2019.
Therapeutic Goods Administration. Guidance 15: Biopharmaceutic studies, Australian Regulatory Guidelines for Prescription Medicines (ARGPM). . 2015 https://www.tga.gov.au/guidance-15-biopharmaceutic-studies. Accessed 28 Nov.2019.
Cardot J-M, Garcia Arieta A, Paixao P, Tasevska I, Davit B. Implementing the biopharmaceutics classification system in drug development: reconciling similarities, differences, and shared challenges in the EMA and US-FDA-recommended approaches. AAPS J. 2016;18(4):1039–46.
Davit BM, Kanfer I, Tsang YC, Cardot J-M. BCS biowaivers: similarities and differences among EMA, FDA, and WHO requirements. AAPS J. 2016;18(3):612–8.
Van Oudtshoorn JE, García-Arieta A, Santos GML, Crane C, Rodrigues C, Simon C, et al. A survey of the regulatory requirements for BCS-based biowaivers for solid oral dosage forms by participating regulators and organisations of the international generic drug regulators programme. J Pharm Pharm Sci. 2018;21(1):27–37.
Loebenberg R. The BCS and biowaivers a global overview. 2015. On-line available at http://pqri.org/wp-content/uploads/2017/02/3-Loebenerg-v2.pdf. Last accessed 28Nov.2019.
Diaz DA, Colgan ST, Langer CS, Bandi NT, Likar MD, Van Alstine L. Dissolution similarity requirements: how similar or dissimilar are the global regulatory expectations? AAPS J. 2016;18(1):15–22.
Pharmaceuticals and Medical Devices Agency (PMDA). Guidance on bioequivalence tests for manufacturing process changes of solid oral dosage forms. Ministry of Health and Welfare. Japan: Yakuji Nippo Ltd. 2013.
Pharmaceuticals and Medical Devices Agency (PMDA). Japanese Pharmacopeia 17. Ministry of Health and Welfare. Japan: Yakuji Nippon Ltd. 2017.
Pharmaceuticals and Medical Devices Agency (PMDA). Guideline for bioequivalence studies for formulation changes of oral solid dosage forms. Ministry of Health and Welfare. Japan: Yakuji Nippo Ltd. 2012.
Pharmaceuticals and Medical Devices Agency (PMDA). Guideline for bioequivalence studies of generic products. Ministry of Health and Welfare. Japan: Yakuji Nippo Ltd. 2012.
U.S. Department of Health and Human Services—Food and Drug Administration CDER. Guidance for industry: SUPAC-MR: modified release solid oral dosage forms: scale-up and postapproval changes: chemistry, manufacturing, and controls; in vitro dissolution testing and in vivo bioequivalence documentation. 1997.
Bou-Chacra N, Melo KJC, Morales IAC, Stippler ES, Kesisoglou F, Yazdanian M, et al. Evolution of choice of solubility and dissolution media after two decades of biopharmaceutical classification system. AAPS J. 2017;19(4):989–1001.
U.S. Department of Health and Human Services - Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry - dissolution testing of immediate release solid oral dosage forms. 1997.
National Health Surveillance Agency of Brazil (ANVISA). Resolution RDC no 31. Provides information about the studies of pharmaceutical equivalence and comparative dissolution profile.2010.
Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999;46(3):183–96.
In vitro dissolution profiles similarity assessment in support of drug product quality: what, how, and when. 2019. https://www.pharmacy.umaryland.edu/centers/cersievents/dissolution-similarity. Last accessed 28Nov.2019
Mitra A, Kesisoglou F, Dogterom P. Application of absorption modeling to predict bioequivalence outcome of two batches of etoricoxib tablets. AAPS PharmSciTech. 2015;16(1):76–84.
Suarez-Sharp S, Cohen M, Kesisoglou F, Abend A, Marroum P, Delvadia P, et al. Applications of clinically relevant dissolution testing: workshop summary report. AAPS J. 2018;20(6):93.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abend, A., Xiong, L., Zhang, X. et al. Biowaiver Applications in Support of a Polymorph During Late-Stage Clinical Development of Verubecestat—Current Challenges and Future Opportunities for Global Regulatory Alignment. AAPS J 22, 17 (2020). https://doi.org/10.1208/s12248-019-0396-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0396-9