Abstract
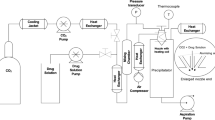
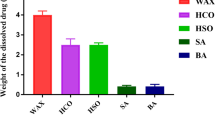
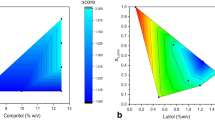
This contribution describes a continuous process for the production of solid lipid nanoparticles (SLN) as drug-carrier systems via hot-melt extrusion (HME). Presently, HME technology has not been used for the manufacturing of SLN. Generally, SLN are prepared as a batch process, which is time consuming and may result in variability of end-product quality attributes. In this study, using Quality by Design (QbD) principles, we were able to achieve continuous production of SLN by combining two processes: HME technology for melt-emulsification and high-pressure homogenization (HPH) for size reduction. Fenofibrate (FBT), a poorly water-soluble model drug, was incorporated into SLN using HME-HPH methods. The developed novel platform demonstrated better process control and size reduction compared to the conventional process of hot homogenization (batch process). Varying the process parameters enabled the production of SLN below 200 nm. The dissolution profile of the FBT SLN prepared by the novel HME-HPH method was faster than that of the crude FBT and a micronized marketed FBT formulation. At the end of a 5-h in vitro dissolution study, a SLN formulation released 92–93% of drug, whereas drug release was approximately 65 and 45% for the marketed micronized formulation and crude drug, respectively. Also, pharmacokinetic study results demonstrated a statistical increase in Cmax, Tmax, and AUC0–24 h in the rate of drug absorption from SLN formulations as compared to the crude drug and marketed micronized formulation. In summary, the present study demonstrated the potential use of hot-melt extrusion technology for continuous and large-scale production of SLN.






Similar content being viewed by others
References
Patil A, Pokharkar V. Single step spray drying method to develop proliposomes for inhalation: a systematic study based on quality by design approach. Pulm Pharmacol Ther. 2014;27:197–207.
Basalious EB, El-Sebaie W, El-Gazayerly O. Application of pharmaceutical QbD for enhancement of the solubility and dissolution of a class II BCS drug using polymeric surfactants and crystallization inhibitors: development of controlled-release tablets. AAPS Pharm Sci Technol. 2011;12:799–810.
Maltesen MJ, Bjerregaard S, Hovgaard L, Havelund S, Weert M. Quality by design spray drying of insulin intended for inhalation. Eur J Pharm Biopharm. 2008;70:828–38.
Yerlikaya F, Ozgen A, Vural I, Guven O, Karaagaoglu E, et al. Development and evaluation of paclitaxel nanoparticles using a quality-by-design approach. J Pharm Sci. 2013;102:3748–61.
US FDA Guidance for industry: PAT—a framework for innovative pharmaceutical development, manufacturing, and quality assurance. Maryland: Silver Spring; 2004.
Hanafy A, Spahn-Langguth H, Vergnault G, Grenier P, Tubic Grozdanis M, et al. Pharmacokinetic evaluation of oral fenofibrate nanosuspensions and SLN in comparison to conventional suspensions of micronized drug. Adv Drug Deliv Rev. 2007;59:419–26.
Rao GC, Kumar MS, Mathivanan N, Rao ME. Nanosuspensions as the most promising approach in nanoparticulate drug delivery systems. Pharmazie. 2004;59:5–9.
Patil H, Kulkarni V, Majumdar S, Repka MA. Continuous manufacturing of solid lipid nanoparticles by hot melt extrusion. Int J Pharm. 2014;471:153–6.
Niu X, Wan L, Hou Z, Wang T, Sun C, et al. Mesoporous carbon as a novel drug carrier of fenofibrate for enhancement of the dissolution and oral bioavailability. Int J Pharm. 2013;452:382–9.
Sanganwar GP, Gupta RP. Dissolution-rate enhancement of fenofibrate by adsorption onto silica using supercritical carbon dioxide. Int J Pharm. 2008;360:213–8.
Wishart DS, Konx C, Guo AC, Shrivastava S, Hassanali M, et al. Drug bank: a comprehensive resource for in silicon drug discovery and exploration. Nucleic Acids Res. 2006;1:D668–72.
Ming-Thau S, Ching-Min Y, Sokoloski TD. Characterization and dissolution of fenofibrate solid dispersion systems. Int J Pharm. 1994;103:137–46.
Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96.
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26.
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33:1043–57.
Breitenbach J. Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm. 2002;54:107–17.
Maniruzzaman M, Boateng JS, Snowde MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN Pharm. 2012;1–9.
Das S, Ng WK, Kanaujia P, Kim S, Tan RB. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: effects of process variables. Colloids Surf B: Biointerfaces. 2011;88:483–9.
Dong Y, Ng WK, Shen S, Kim S, Tan RB. Solid lipid nanoparticles: continuous and potential large-scale nanoprecipitation production in static mixers. Colloids Surf B: Biointerfaces. 2012;94:68–72.
Raza K, Singh B, Singal P, Wadhwa S, Katare OP. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf B: Biointerfaces. 2013;105:67–74.
Shah RM, Malherbe F, Eldridge D, Palombo EA, Harding IH. Physicochemical characterization of solid lipid nanoparticles (SLNs) prepared by a novel microemulsion technique. J Colloid Interface Sci. 2014;428:286–94.
Luo Y, Chen D, Ren L, Zhao X, Qin J. Solid lipid nanoparticles for enhancing vinpocetine's oral bioavailability. J Control Release. 2006;114:53–9.
Wang S, Chen T, Chen R, Hu Y, Chen M, et al. Emodin loaded solid lipid nanoparticles: preparation, characterization and antitumor activity studies. Int J Pharm. 2012;430:238–46.
ICH Q 1 A (R2). Stability testing of new drug substances and products. http://www.emea.eu.int/pdfs/human/ich/273699en.pdf
Hu L, Tang X, Cui F. Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J Pharm Pharmacol. 2004;56:1527–35.
Liu J, Hu W, Chen H, Ni Q, Xu H, et al. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328:191–5.
Rahman Z, Zidan AS, Habib MJ, Khan MA. Understanding the quality of protein loaded PLGA nanoparticles variability by Plackett-Burman design. Int J Pharm. 2010;389(1–2):186–94.
Huang QP, Wang JX, Zhang ZB, Shen ZG, Chen JF, et al. Preparation of ultrafine fenofibrate powder by solidification process from emulsion. Int J Pharm. 2009;368:160–4.
Van Drooge DJ, Hinrichs WL, Frijlink HW. Anomalous dissolution behaviour of tablets prepared from sugar glass-based solid dispersions. J Control Release. 2004;97:441–52.
Mosharraf M, NyatrÖm C. The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. Int J Pharm. 1995;122:35–47.
Müller RH, Peters K. Nanosuspensions for the formulation of poorly soluble drugs: I. Preparation by a size-reduction technique. Int J Pharm. 1998;160:229–37.
Jia Z, Lin P, Xiang Y, Wang X, Wang J, Zhang X, et al. A novel nanomatrix system consisted of colloidal silica and pH-sensitive polymethylacrylate improves the oral bioavailability of fenofibrate. Eur J Pharm Biopharm. 2011;79(1):126–34.
Borkar N, Xia D, Holm R, Gan Y, Mullertz A, Yang M, et al. Investigating the correlation between in vivo absorption and in vitro release of fenofibrate from lipid matrix particles in biorelevant medium. Eur J Pharm Sci. 2014;51:204–10.
Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Ping Gao and Lawrence Yu
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1.20 mb)
Rights and permissions
About this article
Cite this article
Patil, H., Feng, X., Ye, X. et al. Continuous Production of Fenofibrate Solid Lipid Nanoparticles by Hot-Melt Extrusion Technology: a Systematic Study Based on a Quality by Design Approach. AAPS J 17, 194–205 (2015). https://doi.org/10.1208/s12248-014-9674-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9674-8