Abstract
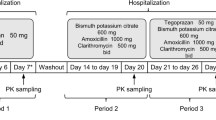
Many orally administered, small-molecule, targeted anticancer drugs, such as dasatinib, exhibit pH-dependent solubility and reduced drug exposure when given with acid-reducing agents. We previously demonstrated that betaine hydrochloride (BHCl) can transiently re-acidify gastric pH in healthy volunteers with drug-induced hypochlorhydria. In this randomized, single-dose, three-way crossover study, healthy volunteers received dasatinib (100 mg) alone, after pretreatment with rabeprazole, and with 1500 mg BHCl after rabeprazole pretreatment, to determine if BHCl can enhance dasatinib absorption in hypochlorhydric conditions. Rabeprazole (20 mg b.i.d.) significantly reduced dasatinib Cmax and AUC0-∞ by 92 and 78%, respectively. However, coadministration of BHCl significantly increased dasatinib Cmax and AUC0-∞ by 15- and 6.7-fold, restoring them to 105 and 121%, respectively, of the control (dasatinib alone). Therefore, BHCl reversed the impact of hypochlorhydria on dasatinib drug exposure and may be an effective strategy to mitigate potential drug-drug interactions for drugs that exhibit pH-dependent solubility and are administered orally under hypochlorhydric conditions.


Similar content being viewed by others
References
Dressman JB, Vertzoni M, Goumas K, Reppas C. Estimating drug solubility in the gastrointestinal tract. Adv Drug Deliv Rev. 2007;59:591–602.
Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28:930–7.
National Center for Health Statistics. Health, United States, 2012: with special feature on emergency care. 2013.
Chin TW, Loeb M, Fong IW. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob Agents Chemother. 1995;39:1671–5.
Eley T, Luo FR, Agrawal S, Sanil A, Manning J, Li T, et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharmacol. 2009;49:700–9.
Zhu L, Persson A, Mahnke L, Eley T, Li T, Xu X, et al. Effect of low-dose omeprazole (20 mg daily) on the pharmacokinetics of multiple-dose atazanavir with ritonavir in healthy subjects. J Clin Pharmacol. 2011;51:368–77.
Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92:203–13.
Smelick GS, Heffron TP, Chu L, Dean B, West DA, Duvall SL, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm. 2013;10:4055–62.
US Food and Drug Administration. Dasatinib (Sprycel) summary basis of approval. 2006.
US Food and Drug Administration. Erlotinib (Tarceva) prescribing information. 2010.
US Food and Drug Administration. Gefitinib (Iressa) prescribing information. 2004.
US Food and Drug Administration. Nilotinib (Tasigna) prescribing information. 2013.
US Food and Drug Administration. Crizotinib (Xalkori) prescribing information. 2013.
Jaruratanasirikul S, Kleepkaew A. Influence of an acidic beverage (Coca-Cola) on the absorption of itraconazole. Eur J Clin Pharmacol. 1997;52:235–7.
Ray JE, Marriott D, Bloch MT, McLachlan AJ. Therapeutic drug monitoring of atazanavir: surveillance of pharmacotherapy in the clinic. Br J Clin Pharmacol. 2005;60:291–9.
Yago MR, Frymoyer AR, Smelick GS, Frassetto LA, Budha NR, Dresser MJ, et al. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Mol Pharm. 2013;10:4032–7.
Michalek W, Semler JR, Kuo B. Impact of acid suppression on upper gastrointestinal pH and motility. Dig Dis Sci. 2011;56:1735–42.
Wedemeyer RS, Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. 2014;37:201–11.
Meyer UA. Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs. Eur J Gastroenterol Hepatol. 1996;8 Suppl 1:S21–5.
Miura M, Satoh S, Tada H, Habuchi T, Suzuki T. Stereoselective metabolism of rabeprazole-thioether to rabeprazole by human liver microsomes. Eur J Clin Pharmacol. 2006;62:113–7.
Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, et al. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14:352–9.
Bristol-Myers Squibb. The effect of omeprazole on the pharmacokinetics of dasatinib (BMS-354825) in healthy subjects. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000–2014. Available from: http://clinicaltrials.gov/show/NCT00655746:NCT00655746. 2009.
Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–78.
Fish MP, Young J, Shah P, Gao Z. The use of experimental design principles in dissolution method development: development of a discriminating dissolution method for Sprycel film-coated tablets. J Pharm Innov. 2009;4:165–73.
van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35:692–706.
Grande E, Kreissl MC, Filetti S, Newbold K, Reinisch W, Robert C, et al. Vandetanib in advanced medullary thyroid cancer: review of adverse event management strategies. Adv Ther. 2013;30:945–66.
US Food and Drug Administration. Vandetanib (Calpresa) summary basis of approval. 2011.
Kantarjian HM, Smith TL, O’Brien S, Beran M, Pierce S, Talpaz M. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-alpha therapy. The Leukemia Service. Ann Intern Med. 1995;122:254–61.
Wang X, Roy A, Hochhaus A, Kantarjian HM, Chen TT, Shah NP. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a Phase III study. Clin Pharmacol. 2013;5:85–97.
Acknowledgments
The authors would like to acknowledge the staff and nurses of the UCSF-CCRC for their assistance in this study. The UCSF-CCRC is supported by an NIH/NCRR grant (UL1 RR0224131). This study was supported by a grant from Genentech, Inc., and M. R. Yago was supported in part by NIH Training Grant T32 GM007175.
Conflict of Interest
L. Z. Benet is a consultant for Genentech, Inc., and X. Ding, B. Dean, L. Salphati, N. Budha, J. Y. Jin, M. J. Dresser, and J. A. Ware are employees of Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yago, M.R., Frymoyer, A., Benet, L.Z. et al. The Use of Betaine HCl to Enhance Dasatinib Absorption in Healthy Volunteers with Rabeprazole-Induced Hypochlorhydria. AAPS J 16, 1358–1365 (2014). https://doi.org/10.1208/s12248-014-9673-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9673-9