Abstract
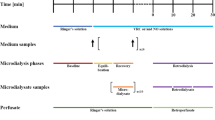
Reliable drug concentration measurements at the target site are increasingly demanded and can be achieved by microdialysis. The aim of this pilot study was to demonstrate the proof of principle of long-term subcutaneous microdialysis in humans. For long-term microdialysis, a special setting implementing both concentric and linear catheters has been developed ensuring good clinical practice compliance, tolerability, and convenience for participants and personnel. As a model compound, moderately lipophilic voriconazole was selected as a well-characterized drug in in vitro microdialysis experiments. Multiple in vivo relative recovery (RR) determinations for microdialysis were performed by retrodialysis during the entire study (n = 48 samples). Continuous microdialysis was successfully applied and well tolerated over 87 h in three adults for the first time. RR revealed low intra-individual (coefficient of variation (CV) = 4.4–12.5%) and inter-individual variability (CV = 4.3–12.5%) across all samples and catheters. Lower RR values were consistently determined for linear catheters. One catheter leakage was managed without an impact on the reliability of the RR values. Overall, RR values were calculated to be 73.3% (linear: CV = 18.5%, n = 23) and 84.9% (concentric: CV = 5.6%, n = 23). Long-term microdialysis application over almost 4 days was feasible by reliable multiple RR (proof of principle), well tolerated, and reduced the burden in humans avoiding several catheter insertions, thereby allowing to monitor concentration–time courses continuously. Moreover, a moderately lipophilic drug has been proven suitable for in vivo microdialysis, as previously suggested by in vitro microdialysis.


Similar content being viewed by others
REFERENCES
Plock N, Kloft C. Microdialysis—theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci. 2005;25(1):1–24.
Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, et al. AAPS-FDA Workshop White Paper: microdialysis principles, application, and regulatory perspectives. J Clin Pharmacol. 2007;47(5):589–603.
Buerger C, Plock N, Dehghanyar P, Joukhadar C, Kloft C. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob Agents Chemother. 2006;50(7):2455–63.
Dehghanyar P, Burger C, Zeitlinger M, Islinger F, Kovar F, Muller M, et al. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob Agents Chemother. 2005;49(6):2367–71.
Gattringer R, Urbauer E, Traunmuller F, Zeitlinger M, Dehghanyar P, Zeleny P, et al. Pharmacokinetics of telithromycin in plasma and soft tissues after single-dose administration to healthy volunteers. Antimicrob Agents Chemother. 2004;48(12):4650–3.
Persky AM, Muller M, Derendorf H, Grant M, Brazeau GA, Hochhaus G. Single- and multiple-dose pharmacokinetics of oral creatine. J Clin Pharmacol. 2003;43(1):29–37.
Traunmuller F, Fille M, Thallinger C, Joukhadar C. Multiple-dose pharmacokinetics of telithromycin in peripheral soft tissues. Int J Antimicrob Agents. 2009;34(1):72–5.
Traunmuller F, Zeitlinger M, Zeleny P, Muller M, Joukhadar C. Pharmacokinetics of single- and multiple-dose oral clarithromycin in soft tissues determined by microdialysis. Antimicrob Agents Chemother. 2007;51(9):3185–9.
Arner P, Bolinder J. Microdialysis of adipose tissue. J Intern Med. 1991;230(4):381–6.
Baumeister FA, Rolinski B, Busch R, Emmrich P. Glucose monitoring with long-term subcutaneous microdialysis in neonates. Pediatrics. 2001;108(5):1187–92.
Bolinder J, Ungerstedt U, Arner P. Microdialysis measurement of the absolute glucose concentration in subcutaneous adipose tissue allowing glucose monitoring in diabetic patients. Diabetologia. 1992;35(12):1177–80.
Bolinder J, Ungerstedt U, Arner P. Long-term continuous glucose monitoring with microdialysis in ambulatory insulin-dependent diabetic patients. Lancet. 1993;342(8879):1080–5.
Edsander-Nord A, Rojdmark J, Wickman M. Metabolism in pedicled and free TRAM flaps: a comparison using the microdialysis technique. Plast Reconstr Surg. 2002;109(2):664–73.
Hashiguchi Y, Sakakida M, Nishida K, Uemura T, Kajiwara K, Shichiri M. Development of a miniaturized glucose monitoring system by combining a needle-type glucose sensor with microdialysis sampling method. Long-term subcutaneous tissue glucose monitoring in ambulatory diabetic patients. Diabetes Care. 1994;17(5):387–96.
Hildingsson U, Sellden H, Ungerstedt U, Marcus C. Microdialysis for metabolic monitoring in neonates after surgery. Acta Paediatr. 1996;85(5):589–94.
Holzinger A, Bonfig W, Kusser B, Eggermann T, Muller H, Munch HG. Use of long-term microdialysis subcutaneous glucose monitoring in the management of neonatal diabetes. A first case report. Biol Neonate. 2006;89(2):88–91.
Horal M, Ungerstedt U, Persson B, Westgren M, Marcus C. Metabolic adaptation in IUGR neonates determined with microdialysis—a pilot study. Early Hum Dev. 1995;42(1):1–14.
Lutgers HL, Hullegie LM, Hoogenberg K, Sluiter WJ, Dullaart RP, Wientjes KJ, et al. Microdialysis measurement of glucose in subcutaneous adipose tissue up to three weeks in type 1 diabetic patients. Neth J Med. 2000;57(1):7–12.
Persson L, Valtysson J, Enblad P, Warme PE, Cesarini K, Lewen A, et al. Neurochemical monitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg. 1996;84(4):606–16.
Hillered L, Valtysson J, Enblad P, Persson L. Interstitial glycerol as a marker for membrane phospholipid degradation in the acutely injured human brain. J Neurol Neurosurg Psychiatry. 1998;64(4):486–91.
Hutchinson PJ, O’Connell MT, Maskell LB, Pickard JD. Monitoring by subcutaneous microdialysis in neurosurgical intensive care. Acta Neurochir Suppl. 1999;75:57–9.
During MJ, Fried I, Leone P, Katz A, Spencer DD. Direct measurement of extracellular lactate in the human hippocampus during spontaneous seizures. J Neurochem. 1994;62(6):2356–61.
Kennergren C, Mantovani V, Lonnroth P, Nystrom B, Berglin E, Hamberger A. Monitoring of extracellular aspartate aminotransferase and troponin T by microdialysis during and after cardioplegic heart arrest. Cardiology. 1999;92(3):162–70.
Konings IR, Engels FK, Sleijfer S, Verweij J, Wiemer EA, Loos WJ. Application of prolonged microdialysis sampling in carboplatin-treated cancer patients. Cancer Chemother Pharmacol. 2009;64(3):509–16.
Kopacz DJ, Bernards CM, Allen HW, Landau C, Nandy P, Wu D, et al. A model to evaluate the pharmacokinetic and pharmacodynamic variables of extended-release products using in vivo tissue microdialysis in humans: bupivacaine-loaded microcapsules. Anesth Analg. 2003;97(1):124–31.
Stahle L, Alm C, Ekquist B, Lundquist B, Tomson T. Monitoring free extracellular valproic acid by microdialysis in epileptic patients. Ther Drug Monit. 1996;18(1):14–8.
Lindberger M, Tomson T, Stahle L. Validation of microdialysis sampling for subcutaneous extracellular valproic acid in humans. Ther Drug Monit. 1998;20(3):358–62.
Simmel F, Kloft C. Microdialysis feasibility investigations with the non-hydrophilic antifungal voriconazole for potential applications in nonclinical and clinical settings. Int J Clin Pharmacol Ther. 2010;48(11):695–704.
World Medical Association. Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. 1964 (amended by the 59th WMA General Assembly, Seoul, October 2008). http://www.wma.net/en/30publications/10policies/b3/index.html. Accessed 27 Oct 2009.
Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95) Step 5. 1996.
Stahle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis. III: Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49(24):1853–8.
Simmel F, Soukup J, Zoerner A, Radke J, Kloft C. Development and validation of an efficient HPLC method for quantification of voriconazole in plasma and microdialysate reflecting an important target site. Anal Bioanal Chem. 2008;392(3):479–88.
Guidance for Industry. Bioanalytical Method Validation. Food and Drug Administration. 2001. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. Accessed 27 October 2009.
Saunte DM, Simmel F, Frimodt-Moller N, Stolle LB, Svejgaard EL, Haedersdal M, et al. In vivo efficacy and pharmacokinetics of voriconazole in an animal model of dermatophytosis. Antimicrob Agents Chemother. 2007;51(9):3317–21.
Araujo BV, Conrado DJ, Palma EC, Dalla Costa T. Validation of rapid and simple LC-MS/MS method for determination of voriconazole in rat plasma. J Pharm Biomed Anal. 2007;44(4):985–90.
Araujo BV, Silva CF, Haas SE, Dalla Costa T. Microdialysis as a tool to determine free kidney levels of voriconazole in rodents: a model to study the technique feasibility for a moderately lipophilic drug. J Pharm Biomed Anal. 2008;47(4–5):876–81.
Joukhadar C, Thallinger C, Poppl W, Kovar F, Konz KH, Joukhadar SM, et al. Concentrations of voriconazole in healthy and inflamed lung in rats. Antimicrob Agents Chemother. 2009;53(6):2684–6.
ACKNOWLEDGMENT
The authors wish to thank Dorothea Frenzel for her excellent technical support during bioanalysis and Matthias Stezycki for illustration of the long-term microdialysis setting.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simmel, F., Kirbs, C., Erdogan, Z. et al. Pilot Investigation on Long-Term Subcutaneous Microdialysis: Proof of Principle in Humans. AAPS J 15, 95–103 (2013). https://doi.org/10.1208/s12248-012-9412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9412-z