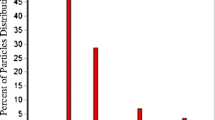
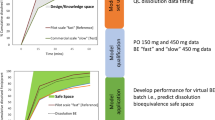
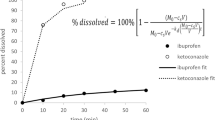
Abstract
In early drug development, the selection of a formulation platform and decisions on formulation strategies have to be made within a short timeframe and often with minimal use of the active pharmaceutical ingredient (API). The current work evaluated the various physicochemical parameters required to improve the prediction accuracy of simulation software for immediate release tablets in early drug development. DDDPlus™ was used in simulating dissolution test profiles of immediate release tablets of ritonavir and all simulations were compared with experimental results. The minimum data requirements to make useful predictions were assessed using the ADMET predictor (part of DDDPlus) and Chemicalize (an online resource). A surfactant model was developed to estimate the solubility enhancement in media containing surfactant and the software’s transfer model based on the USP two-tiered dissolution test was assessed. One measured data point was shown to be sufficient to make predictive simulations in DDDPlus. At pH 2.0, the software overestimated drug release while at pH 1.0 and 6.8, simulations were close to the measured values. A surfactant solubility model established with measured data gave good dissolution predictions. The transfer model uses a single-vessel model and was unable to predict the two in vivo environments separately. For weak bases like ritonavir, a minimum of three solubility data points is recommended for in silico predictions in buffered media. A surfactant solubility model is useful when predicting dissolution behavior in surfactant media and in silico predictions need measured solubility data to be predictive.







Similar content being viewed by others
References
NORVIR (ritonavir) Label – FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209512lbl.pdf. Accessed 6 Nov 2018.
Rock BM, Hengel SM, Rock DA, Weinkers LC, Kunze KL. Characterization of ritonavir-mediated inactivation of cytochrome P450 3A4. Mol Pharmacol. 2014;86(6):665–74. https://doi.org/10.1124/mol.114.094862.
https://chemaxon.com/products/chemicalize. Accessed 10 Feb 2017.
Law D, Krill S, Schmitt EA, Fort JJ, Qui Y, Wang W, et al. Physicochemical considerations in the preparation of amorphous ritonavir-poly(ethylene glycol) 8000 solid dispersions. J Pharm Sci. 2001;90(8):1015–25.
Xu H, Vela S, Shi Y, Marroum P, Gao P. In vitro characterization of ritonavir drug products and correlation to human in vivo performance. Mol Pharm. 2017;14:3801–14.
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29(3–4):278–87.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pham Res. 1995;12:413–20.
Ilevbare GA, Taylor LS. Liquid-liquid phase separation in highly supersaturated aqueous solutions of poorly water-soluble drugs: implications for solubility enhancing formulations. Cryst Growth Des. 2013;13(4):1497–509.
Hewitt M, Cronin MTD, Enoch SJ, Madden JC, Roberts DW, Dearden JC. In silico prediction of aqueous solubility: the solubility challenge. J Chem Inf Model. 2009;49:2572–87.
Suarez-Sharp S, Cohe M, Kesisoglou F, Abend A, Marroum P, Delvadia P, et al. Applications of clinically relevant dissolution testing: workshop summary report. AAPS J. 2018;20:9. https://doi.org/10.1208/s12248-018-0252-3.
Löbenberg R, Amidon G. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur J Pharm Biopharm. 2000;50(1):3–12.
Duque MD, Issa MG, Silva DA, Kakuda BAS, Rodrigues LNC, Löbenberg R, et al. Intrinsic dissolution simulation of highly and poorly soluble drugs for BCS solubility classification. Dissolut Technol. 2017;24(4):6–11.
Almukainzi M, Okumu A, Wei H, Löbenberg R. Simulation of in vitro dissolution behavior using DDDPlus™. AAPS PharmSciTech. 2015;16(1):217–21.
Uebbing L, Klumpp L, Webster GK, Löbenberg R. Justification of disintegration testing beyond current FDA criteria using in vitro and in silico models. Drug Des Dev Ther. 2017;11(11):1163–74.
Abend A, Curran D, Kuiper J, Lu X, Li H, Hermans A, et al. Dissolution testing in drug product development: workshop summary report. AAPS J. 2019;21(2):21. https://doi.org/10.1208/s12248-018-0288-4.
The United States Pharmacopeia 37. The National Formulary 32, vol. 1. North Bethesda: United States Pharmacopeial Convention; 2014. p. 1443–4.
Bou-Chacra N, Melo KJC, Morales IAC, Stippler ES, Kesisoglou F, Yazdanian M, et al. Evolution of choice of solubility and dissolution media after two decades of biopharmaceutical classification system. AAPS J. 2017;19(4):989–1001.
Simulations Plus Inc. The DDDPlus user manual is available within the DDDPlusTM software program; 2016.
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263–71.
Palmgrén JJ, Mönkkönen J, Korjamo T, Hassinen A, Auriola S. Drug adsorption to plastic containers and retention of drugs in cultured cells under in vitro conditions. Eur J Pharm Biopharm. 2006;64:369–78.
Webster GK, Jackson JD, Bell RG. Poorly soluble drugs dissolution and drug release. Pan Stanford series on pharmaceutical analysis. Pan Stanford Series on Pharmaceutical Analysis 2017;1:6–60, 209–231.
FDA-CDER Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. https://www.fda.gov/downloads/Drugs/Guidances/ucm070246.pdf. Accessed 15 Jan 2018.
Pandey P, Hamey R, Bindra DS, Huang Z, Mathias N, Eley T, et al. From bench to humans: formulation development of a poorly water soluble drug to mitigate food effect. AAPS PharmSciTech. 2014;15(2):407–16.
Feng D, Peng T, Huang Z, Singh V, Shi Y, Wen T, et al. Polymer–surfactant system based amorphous solid dispersion: precipitation inhibition and bioavailability enhancement of itraconazole. Pharmaceutics. 2018;10(2):53. https://doi.org/10.3390/pharmaceutics10020053.
Stojančević M, Pavlović N, Goločorbin-Kon S, Mikov M. Application of bile acids in drug formulation and delivery. Front Life Sci. 2013;7(3–4):112–22.
Noory C, Tran N, Ouderkirk L, Shah V. Steps for development of a dissolution test for sparingly water-soluble drug products. Dissolut Technol. 2000;7(1):16–8.
Jinno J, Kamada N, Miyake M, Yamada K, Mukai T, Odomi M, et al. In vitro-in vivo correlation for wet-milled tablet of poorly water-soluble cilostazol. J Control Release. 2008;130(1):29–37.
Zuo J, Gao Y, Bou-Chacra N, Löbenberg R. Evaluation of the DDSolver software applications. Biomed Res Int. 2014;2014:1–9. https://doi.org/10.1155/2014/204925.
Amaral JF, Thompson WR, Caldwell MD, Martin HF, Randall HT. Prospective hematologic evaluation of gastric exclusion surgery for morbid obesity. Ann Surg. 1985;201(2):186–93.
Silva DA, Duque MD, Davies NM, Löbenberg R, Ferraz HG. Application of in silico tools in clinical practice using ketoconazole as a model drug. J Pharm Pharm Sci. 2018;21(1s):242s–53s. https://doi.org/10.18433/jpps30227.
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci. 2014;57:342–66.
Nguyen MA, Flanagan T, Brewster M, Kesisoglou F, Beato S, Biewenga J, et al. Survey on IVIVC/IVIVR development in the pharmaceutical industry – past experience and current perspectives. Eur J Pharm Sci. 2017;102:1–13.
Lu E, Li S, Wang Z. Biorelevant test for supersaturable formulation. Asian J Pharm Sci. 2017;12:9–20.
Tsume Y, Mudie DM, Langguth P, Amidon GE, Amidon GL. The biopharmaceutics classification system: subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur J Pharm Sci. 2014;57:152–16.
Xu H, Krakow S, Shi Y, Rosenberg J, Gao P. In vitro characterization of ritonavir formulations and correlation to in vivo performance in dogs. Eur J Pharm Sci. 2018;115:286–95.
Okumu A, DiMaso M, Löbenberg R. Computer simulations using GastroPlus™ to justify a biowaiver for etoricoxib solid oral drug products. Eur J Pharm Biopharm. 2009;72(1):91–8. https://doi.org/10.1016/j.ejpb.2008.10.019.
Acknowledgments
The authors would like to thank Simulations Plus, the Drug Development and Innovation Centre of the University of Alberta and AbbVie for their support.
Juliet Obianuju Njoku, Daniela Amaral Silva are graduate students and Raimar Löbenberg is a professor at the Faculty of Pharmacy and Pharmaceutical Sciences at the University of Alberta. Dwaipayan Mukherjee and Gregory Webster are employees of AbbVie and may hold AbbVie stocks or options. Abbvie jointly participated in the interpretation of data, writing, reviewing and approving the publication. The presentation contains no proprietary AbbVie data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Njoku, J.O., Amaral Silva, D., Mukherjee, D. et al. In silico Tools at Early Stage of Pharmaceutical Development: Data Needs and Software Capabilities. AAPS PharmSciTech 20, 243 (2019). https://doi.org/10.1208/s12249-019-1461-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1461-5