Abstract
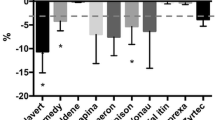
Pharmaceutical excipients contain reactive groups and impurities due to manufacturing processes that can cause decomposition of active drug compounds. The aim of this investigation was to determine if commercially available oral disintegrating tablet (ODT) platforms induce active pharmaceutical ingredient (API) degradation. Benzocaine was selected as the model API due to known degradation through ester and primary amino groups. Benzocaine was either compressed at a constant pressure, 20 kN, or at pressure necessary to produce a set hardness, i.e., where a series of tablets were produced at different compression forces until an average hardness of approximately 100 N was achieved. Tablets were then stored for 6 months under International Conference on Harmonization recommended conditions, 25°C and 60% relative humidity (RH), or under accelerated conditions, 40°C and 75% RH. Benzocaine degradation was monitored by liquid chromatography–mass spectrometry. Regardless of the ODT platform, no degradation of benzocaine was observed in tablets that were kept for 6 months at 25°C and 60% RH. After storage for 30 days under accelerated conditions, benzocaine degradation was observed in a single platform. Qualitative differences in ODT platform behavior were observed in physical appearance of the tablets after storage under different temperature and humidity conditions.





Similar content being viewed by others
REFERENCES
Fu YR, Yang SC, Jeong SH, Kimura S, Park K. Orally fast disintegrating tablets: developments, technologies, taste-masking and clinical studies. Crit Rev Ther Drug. 2004;21(6):433–75.
Douroumis D. Orally disintegrating dosage forms and taste-masking technologies; 2010. Expert Opin Drug Deliv. 2011;8(5):665–75.
Montgomery W, Treuer T, Karagianis J, Ascher-Svanum H, Harrison G. Orally disintegrating olanzapine review: effectiveness, patient preference, adherence, and other properties. Patient Prefer Adherence. 2012;6:109–25.
Navarro V. Improving medication compliance in patients with depression: use of orodispersible tablets. Adv Ther. 2010;27(11):785–95.
US FDA. Guidance for industry orally disintegrating tablets. Silver Spring, MD: Office of Pharmaceutical Science in the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration; 2008.
Wu Y, Levons J, Narang AS, Raghavan K, Rao VM. Reactive impurities in excipients: profiling, identification and mitigation of drug–excipient incompatibility. AAPS PharmSciTech. 2011;12(4):1248–63.
Prasad A, Langley N. The effect of secondary oxidation impurities in PVP on API stability. Tarrytown, NY: BASF Corporation
ICH. Stability testing of new drug substances and products Q1A(R2). Step 4: International Conference on the Harmonisation of Technical Requirements for Registration of Pharmaceutical for Human Use; 2003.
Branch SK. Guidelines from the International Conference on Harmonisation (ICH). J Pharm Biomed Anal. 2005;38(5):798–805.
Rumbelow S, Brown JC, editors. Investigations into drug stability using LC-MS-MS data and statistical data processing. 57th American Society of Mass Spectrometry, Philadelphia, USA; 2009.
Narang PK, Bird G, Crouthamel WG. High-performance liquid chromatographic assay for benzocaine and p-aminobenzoic acid including preliminary stability data. J Pharm Sci. 1980;69(12):1384–7.
del Barrio MA, Hu J, Zhou P, Cauchon N. Simultaneous determination of formic acid and formaldehyde in pharmaceutical excipients using headspace GC/MS. J Pharm Biomed Anal. 2006;41(3):738–43.
Waterman KC, Arikpo WB, Fergione MB, Graul TW, Johnson BA, Macdonald BC, et al. N-Methylation and N-formylation of a secondary amine drug (varenicline) in an osmotic tablet. J Pharm Sci. 2008;97(4):1499–507.
Crowley PJ, Martini LG. Effects of excipients on the stability of medicinal products. Chem Today. 2010;28(5 Supplement):VII–XIII.
El-Banna HM, Daabis NA, El-Fattah SA. Aspirin stability in solid dispersion binary systems. J Pharm Sci. 1978;67(11):1631–3.
Wirth DD, Baertschi SW, Johnson RA, Maple SR, Miller MS, Hallenbeck DK, et al. Maillard reaction of lactose and fluoxetine hydrochloride, a secondary amine. J Pharm Sci. 1998;87(1):31–9.
George RC, Barbuch RJ, Huber EW, Regg BT. Investigation into the yellowing on aging of Sabril® tablet cores. Drug Dev Ind Pharm. 1994;20:3023–32.
ACKNOWLEDGMENTS
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06 RR15482 from the National Centre for Research Resources, NIH. This research was funded in part by Roquette America, Inc. The authors would like to thank Dr. Jerry White and Bryan Zahakaylo of UIC-RRC Mass Spectrometry Facility for significant help with LC-MS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Köllmer, M., Popescu, C., Manda, P. et al. Stability of Benzocaine Formulated in Commercial Oral Disintegrating Tablet Platforms. AAPS PharmSciTech 14, 1333–1340 (2013). https://doi.org/10.1208/s12249-013-0015-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-0015-5