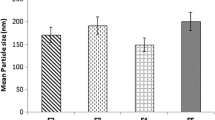
Abstract
The aim of the present study was to investigate the feasibility of the inclusion of a water-insoluble drug (diazepam, DZ) into solid lipid nanoparticles (SLNs), which offer combined advantages of rapid onset and prolonged release of the drug. This work also describes a new approach to prepare suppositories containing DZ-loaded SLN dispersions, as potential drug carrier for the rectal route. Modified high-shear homogenization and ultrasound techniques were employed to prepare SLNs. The effect of incorporation of different concentrations of Compritol® ATO 888 or Imwitor® 900K and Poloxamer 188 or Tween 80 was investigated. Results showed that varying the type or concentration of lipid matrix or surfactant had a noticeable influence on the entrapment efficiencies, particle size, and release profiles of prepared SLNs. Differential scanning calorimetry and X-ray diffraction measurements showed that the majority of SLNs possessed less ordered arrangements of crystals than the corresponding bulk lipids, which was favorable for increasing the drug loading capacity. Transmission electron microscopy and laser diffractometry studies revealed that the prepared nanoparticles were round and homogeneous and 60% of the formulations were less than 500 nm. Additionally, SLN formulations showed significant (P < 0.05) prolonged release than DZ solution. The subsequent step encompassed the preparation and evaluation of SLN-based suppositories utilizing SLN formulations that illustrated optimal release profiles. The in vitro release of DZ from the suppositories prepared using DZ-loaded SLN dispersions (equivalent to 2 mg DZ) was significantly (P < 0.05) extended compared to suppositories containing 2 mg DZ free drug.







Similar content being viewed by others
References
B. Alldredge, A. Gelb, S. Isaacs, M. Corry, F. Allen, S. Ulrich, M. Gottwald, N. O’Neil, J. Neuhaus, M. Segal, and D. Lowenstein. A comparison of lorazepam, DZ, and placebo for the treatment of out-of-hospital status epilepticus. N. Engl. J. Med. 345:631–637 (2001).
C. O’Dell. What do we tell parents of a child with simple or complex febrile seizures? In T. Z. Baram, and S. Shinnar (eds.), Febrile seizures, Academic, San Diego, 2002, pp. 305–316.
C. O’Dell, S. Shinnar, K. Ballaban-Gil, M. Hornick, M. Sigalova, H. Kang, and S. Moshé. Rectal DZ gel in the home management of seizures in children. Pediatr. Neurol. 33:166–172 (2005).
J. O. McNamara. Drugs effective in the therapy of the epilepsies. In A. G. Gilman (ed.), The pharmacological basis of therapeutics, McGraw Hill, New York, 1996, pp. 478–485.
L. Li, I. Nandi, and K. Kim. Development of an ethyl laurate-based microemulsion for rapid-onset intranasal delivery of DZ. Int. J. Pharm. 237:77–85 (2002).
R. Kriel, J. Cloyd, J. Pellock, W. Mitchell, J. Cereghino, and N. Rosman. Rectal DZ gel for treatment of acute repetitive seizures. Pediatr. Neurol. 20:282–288 (1999).
M. Carceles, A. Ribó, R. Dávalos, T. Martinez, and J. Hernández. Effect of DZ on adenosine 3, 5-cyclic monophosphate (cAMP) plasma levels in anesthetized patients. Clin. Ther. 26:737–743 (2004).
R. H. Müller, K. Mader, and S. Gohla. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur. J. Pharm. Biopharm. 50:161–177 (2000).
W. Mehnert, and K. Mader. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug. Deliv. Rev. 472–3:165–196 (2001).
K. Parfitt. Martindale, the complete drug reference, 32rd ed., Pharmaceutical Press, London, 1999, pp. 661–668.
A. MacDonald, A. Michaelis, and B. Senkowski. Analytical profiles of drug substances and excipients, 1:Academic, San Diego, 1972, pp. 79–99.
M. Sznitowska, M. Gajewska, S. Janicki, A. Radwanska, and G. Lukowski. Bioavailability of DZ from aqueous-organic solution, submicron emulsion and solid lipid nanoparticles after rectal administration in rabbits. Eur. J. Pharm. Biopharm. 52:159–163 (2001).
J. Cloyd, R. Lalonde, T. Beniak, and G. Novack. A single blind, crossover comparison of the pharmacokinetics and cognitive effects of a new rectal DZ gel with intravenous DZ. Epilepsia. 39:520–526 (1998).
D. Hou, C. Xie, K. Huang, and C. Zhu. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials. 24:1781–1785 (2003).
E. B. Souto, S. A. Wissing, C. M. Barbosa, and R. H. Müller. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 278:71–77 (2004).
L. H. Reddy, and R. S. Murthy. Etoposide-loaded nanoparticles made from glyceride lipids: formulation, characterization, in vitro drug release, and stability evaluation. AAPS PharmSciTech. 6:(2):Article 24 (2005).
B. Kim, K. Na, and H. Choi. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur. J. Pharm. Sci. 24:199–205 (2005).
A. zur Muhlen, C. Schwarz, and W. Mehnert. Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur. J. Pharm. Biopharm. 452:149–155 (1998).
S. A. Wissing, O. Kayser, and R. H. Müller. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug. Deliv. Rev. 56:1257–1272 (2004).
K. Manjunath, and V. Venkateswarlu. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J. Control. Rel. 107:215–228 (2005).
O. N. El-Gazayerly, and A. H. Hikal. Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int. J. Pharm. 158:121–127 (1997).
G. N. Devaraj, S. R. Parakh, R. Devraj, S. S. Apte, B. R. Rao, and D. Rambhau. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Colloid. Interface Sci. 251:360–365 (2002).
Y. Luo, D. Chen, L. Ren, X. Zhao, and J. Qin. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J. Control. Release. 114:53–59 (2006).
J. Hanaee, Y. Javadzadeh, S. Taftachi, D. Farid, and A. Nokhodchi. The role of various surfactants on the release of salbutamol from suppositories. IL FARMACO. 59:903–906 (2004).
K. Westesen. Particles with modified physico-chemical properties, their preparation and uses, US Patent 6, 197, 349 (2001).
Y. Li, L. Dong, A. Jia, X. Chang, and H. Xue. Preparation and characterization of solid lipid nanoparticles loaded traditional Chinese medicine. Int. J. Biol. Macromol. 38:296–299 (2006).
V. Venkateswarlu, and K. Manjunath. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Control. Release. 95:627–638 (2004).
M. A. Casadei, F. Cerreto, S. Cesa, M. Giannuzzo, M. Feeney, C. Marianecci, and P. Paolicelli. Solid lipid nanoparticles incorporated in dextran hydrogels: a new drug delivery system for oral formulations. Int. J. Pharm. 325:140–146 (2006).
D. Quintanar-Guerrero, D. Tamayo-Esquivel, A. Ganem-Quintanar, E. Allémanna, and E. Doelker. Adaptation and optimization of the emulsification–diffusion technique to prepare lipidic nanospheres. Eur. J. Pharm. Sci. 26:211–218 (2005).
V. Jenning, A. F. Thunemann, and S. H. Gohla. Characterization of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 199:167–177 (2000).
A. zur Muhlen, and W. Mehnert. Drug release mechanism of prednisolone loaded solid lipid nanoparticles. Pharmazie. 53:552–555 (1998).
C. Schwarz, W. Mehnert, and R. H. Müller. Influence of production parameters of solid lipid nanoparticles (SLN) on the suitability for intravenous injection. Eur. J. Pharm. Biopharm. 40:24S (1994).
S. Lim, and C. Kim. Formulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acid. Int. J. Pharm. 243:135–146 (2002).
R. C. Rowe, P. J. Sheskey, and P. J. Weller. Handbook of pharmaceutical excipients, 4th ed., American Pharmaceutical Association, Pharmaceutical Press, London, 2003.
H. Bunjes, K. Westesen, and M. H. J. Koch. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 129:159–173 (1996).
R. Cavalli, O. Caputo, M. Carlotti, M. Trotta, C. Scarnecchia, and M. R. Gasco. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int. J. Pharm. 148:47–54 (1997).
P. Chattopadhyay, B. Y. Shekunov, D. Yimb, D. Cipolla, B. Boyd, and S. Farr. Production of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx system. Adv. Drug. Deliv. Rev. 59:444–453 (2007).
M. Gibaldi, and S. Feldman. Mechanisms of surfactant effects on drug absorption. J. Pharm. Sci. 59:579–589 (1970).
M. Rieger. Surfactants. In H. Lieberman, M. Rieger, and G. Banker (eds.), Pharmaceutical dosage forms: disperse systems, 1:Marcel Dekker, New York, 1988, pp. 285–366.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelbary, G., Fahmy, R.H. Diazepam-Loaded Solid Lipid Nanoparticles: Design and Characterization. AAPS PharmSciTech 10, 211–219 (2009). https://doi.org/10.1208/s12249-009-9197-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9197-2