Abstract
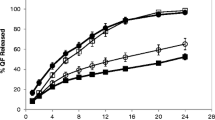
The purpose of this research was to design oral controlled release (CR) matrix tablets of zidovudine (AZT) using hydroxypropyl methylcellulose (HPMC), ethyl cellulose (EC) and carbopol-971P (CP) and to study the effect of various formulation factors on in vitro drug release. Release studies were carried out using USP type 1 apparatus in 900 ml of dissolution media. Release kinetics were analyzed using zero-order, Higuchi’s square root and Ritger–Peppas’ empirical equations. Release rate decreased with increase in polymer proportion and compression force. The release rate was lesser in formulations prepared using CP (20%) as compared to HPMC (20%) as compared to EC (20%). No significant difference was observed in the effect of pH of dissolution media on drug release from formulations prepared using HPMC or EC, but significant difference was observed in CP based formulations. Decrease in agitation speed from 100 to 50 rpm decreased release rate from HPMC and CP formulations but no significant difference was observed in EC formulations. Mechanism of release was found to be dependent predominantly on diffusion of drug through the matrix than polymer relaxation incase of HPMC and EC formulations, while polymer relaxation had a dominating influence on drug release than diffusion incase of CP formulations. Designed CR tablets with pH independent drug release characteristics and an initial release of 17–25% in first hour and extending the release up to 16–20 h, can overcome the disadvantages associated with conventional tablets of AZT.







Similar content being viewed by others
REFERENCES
S. F. Anthony, and H. L. Clifford. Human immunodeficiency virus (HIV) disease: AIDS and related disorders. In: E. Braunwald, A. S. Fauci, D. L. Kasper, S. L. Hauser, D. L. Longo, and J. L. Jameson (eds.), Harrison’s Principles of Internal Medicine, McGraw-Hill, New York, USA, 2001, pp. 1852–1913.
O. L. Laskin, P. de Miranda, and M. R. Blum. Azidothymidine steady-state pharmacokinetics in patients with AIDS and AIDS-related complex. J. Infect. Dis 159 (no. 4): 745–747 (1989).
S. Chitnis, D. Mondal, and K. C. Agrawal. Zidovudine (AZT) treatment suppresses granulocyte–monocyte colony stimulating factor receptor type alpha (GM-CSFR alpha) gene expression in murine bone marrow cells. Life Sci 12:967–978 (2002).
P. Chariot, I. Drogou, I. de Lacroix-Szmania, M. C. Eliezer-Vanerot, B. Chazaud, A. Lombes, A. Schaeffer, and E. S. Zafrani. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J. Hepatol 30 (no. 1): 156–160 (1999).
M. C. Re, I. Bon, P. Monari, R. Gorini, P. Schiavone, D. Gibellini, and M. La Placa. Drug failure during HIV-1 treatment. New perspectives in monitoring drug resistance. New Microbiol 26 (no. 4): 405–413 (2003).
Y. W. Chien. Novel drug delivery systems. In Y. W. Chien (ed.), Oral Drug Delivery and Delivery Systems, Marcel Dekker, New York, USA, 1992, pp. 139–196.
S. P. Vyas, and R. K. Khar. Controlled drug delivery: Concepts and advances. In S. P. Vyas, and R. K. Khar (eds.), Controlled oral administration, Vallabh Prakashan, Delhi, India, 2002, pp. 155–195.
R. K. V. Ranga, D. K. Padmalatha, and B. Buri. Cellulose matrices for zero-order release of soluble drugs. Drug Dev. Ind. Pharm 14:2299–2320 (1988).
I. Zabeed, B. Almas, and A. Muhammad. Controlled release naproxen using micronized ethyl cellulose by wet-granulation and solid-dispersion method. Drug Dev. Ind. Pharm 28 (no. 2):129–134 (2002).
M. Riikka, S. Eero, K. Ossi, P. Heli, N. Riku, L. Marko, and K. Jarkko. Controlled release of saccharides from matrix tablets. Eur. J. Pharm. Biopharm 62:163–170 (2006).
A. B. Silvina, C. L. Marcia, and J. S. Claudio. Swellable matrices for the controlled-release of diclofenac sodium. Formulation and in vitro studies. Pharm. Dev. Tech 9 (no. 1): 75–83 (2004).
T. A. Manuel, and V. R. Leopoldo. Effect of formulation and process variables on the release behavior of amoxicillin matrix tablets. Drug Dev. Ind. Pharm 30 (no. 8): 901–908 (2004).
R. W. Korsenmeyer, and N. A. Peppas. Macromolecular and modeling aspects of swelling-controlled systems. In S. Z. Mansdorf, and T. J. Roseman (eds.), Controlled Release Delivery Systems, Marcel Dekker, New York, USA, 1983, p. 77.
B. J. Lee, S. G. Ryu, and J. H. Cui. Formulation and release characteristics of hydroxypropyl methylcellulose matrix tablet containing melatonin. Drug Dev. Ind. Pharm 25:493–501 (1999).
C. L. Vargas, and E. S. Ghaly. Kinetic release of theophylline from hydrophilic swellable matrices. Drug Dev. Ind. Pharm 25:1045–1050 (1999).
M. V. Velasco, J. L. Ford, P. Rowe, and A. R. Rajabi-Siahboomi. Influence of drug:hydroxypropyl methylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC matrices. J. Control. Rel 57:75–85 (1999).
C. Sajeev, and R. N. Saha. Formulation and comparative evaluation of controlled release diclofenac tablets prepared by matrix embedding technique, membrane barrier technique and combination of the two. Drug Dev. Res 53 (no. 1): 1–8 (2001).
P. Jelena, D. Zorica, J. Milica, and I. Svetlana. An investigation into the factors influencing drug release from hydrophilic matrix tablets based on novel carbomer polymers. Drug Delivery 11:59–65 (2004).
G. S. Rekhi, and S. S. Jambhekar. Ethyl cellulose—a polymer review. Drug Dev. Ind. Pharm 21:61–77 (1995).
P. R. Katikaneni, S. M. Upadrashta, S. H. Neau, and A. K. Mitra. Ethylcellulose matrix controlled release tablets of a water-soluble drug. Int. J. Pharm 123:119–125 (1995).
S. H. Neau, M. A. Howard, J. S. Claudius, and D. R. Howard. The effect of the aqueous solubility of xanthine derivatives on the release mechanism from ethylcellulose matrix tablets. Int. J. Pharm 179:97–105 (1999).
K. Atul, K. T. Ashok, K. J. Narendra, and J. Subheet. Formulation and in vitro, in vivo evaluation of extended-release matrix tablet of zidovudine: Influence of combination of hydrophilic and hydrophobic matrix formers. AAPS Pharm. Sci. Tech. 7(1) (2006) (article 1).
H. A. Benghuzzi, R. M. Barbaro, and P. K. Bajpai. In vitro release of azidothymidine (AZT) by ceramic drug delivery systems. Biomed. Sci. Instrum 26:151–156 (1990).
H. A. Benghuzzi. Long-term sustained delivery of 3’-azido-2’,3’-dideoxythymidine in vivo by means of HA and TCP delivery devices. Biomed. Sci. Instrum 36:343–348 (2000).
R. R. Punna, and R. N. Saha. A new rapid, simple and validated UV spectrophotometric method for estimation of zidovudine in bulk, formulations and dissolution samples. AAPS J. 6(4) (2004) (Abstract T3054).
S. Li, Y. Shen, W. Li, and X. Hao. A common profile for polymer-based controlled release and its logical interpretation to general release process. J. Pharm. Pharmaceut. Sci 9:238–244 (2006).
R. L. Ritger, and N. A. Peppas. A simple equation for the description of solute release. II. Fickian and anomalous release from swellable devices. J. Control. Rel 5:37–42 (1987).
P. Costa, and J. M. S. Lobo. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci 13:123–133 (2001).
A. A. S. Araujo, S. Stropirtis, L. P. Mercuri, F. M. S. Carvalho, F. M. dos Santos, and J. R. Matos. Thermal analysis of the antiretroviral Zidovudine (AZT) and evaluation of the compatibility with excipients used in solid dosage forms. Int. J. Pharm 260:303–314 (2003).
J. L. Ford, M. H. Rubunstein, and J. E. Hogan. Propranolol hydrochloride and aminophylline release from matrix tablets containing hydroxypropyl methylcellulose. Int. J. Pharm 24:339–350 (1985).
M. A. Anjali, H. N. Steven, and L. B. Peter. Wet granulation fine particle ethyl cellulose tablets: Effect of production variables and mathematical modeling of drug release. AAPS PharmSci. 5(2):article 13 (2003).
H. Kim, and R. Fassihi. Application of binary polymer system in drug release rate modulation. 2. Influence of formulation variables and hydrodynamic conditions on release kinetics. J. Pharm. Sci 86 (no. 3): 323–328 (1997).
B. P. Marcos, J. L. Ford, D. J. Armstrong, P. N. C. Elliott, C. Rostron, and J. E. Hogan. Influence of pH on the release of propranolol hydrochloride from matrices containing HPMC K4 M and Carbopol 974. J. Pharm. Sci 85 (no. 3): 330–334 (1996).
F. Atsuko, F. Ryuta, Y. Yorinobu, and S. Hisakazu. Analysis of the release process of phenylpropanolamine hydrochloride from ethylcellulose matrix granules III.1) Effects of the dissolution condition on the release process. Chem. Pharm. Bull 54 (no. 8): 1091–1096 (2006).
N. R. Maichel, L. S. Roger, and B. S. Joseph. The effect of formulation composition and dissolution parameters on the gel strength of controlled release hydrogel tablets. Pharm. Dev. Tech 6 (no. 4): 583–593 (2001).
ACKNOWLEDGEMENTS
The authors are grateful to Strides Arcolab Limited, Bangalore, India, for generous gift samples of AZT and IPCA laboratories, Mumbai, India, for providing gift samples of HPMC, EC and CP. The authors wish to thank University Grants Commission, New Delhi, India, for funding the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravi, P.R., Kotreka, U.K. & Saha, R.N. Controlled Release Matrix Tablets of Zidovudine: Effect of Formulation Variables on the In Vitro Drug Release Kinetics. AAPS PharmSciTech 9, 302–313 (2008). https://doi.org/10.1208/s12249-007-9030-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-007-9030-8